Abstract
We have used V79MZ hamster lung fibroblasts stably transfected with human cytochrome P450-1A1 (hCYP1A1; cell line designated V79MZh1A1) or P450-1B1 (hCYP1B1; cell line designated V79MZh1B1) alone, or in combination with human GST alpha-1 (hGSTA1), in order to examine GST protection against cytotoxicity and mutagenicity of dibenzo[a,l]pyrene (DBP) and the intermediate dihydrodiol metabolite (+/−)-DBP-11,12-dihydrodiol (DBPD). At comparable expression levels of hCYP1A1 and hCYP1B1, both DBP and DBPD were more cytotoxic in V79MZ1A1 (IC50 = 2.7 nM and 0.7 nM, respectively) than in V79MZh1B1 (IC50 = 6.0 nM and 4.8 nM, respectively). In contrast, both DBP and DBPD were 2-fold to 4-fold more mutagenic in V79MZh1B1 than in V79MZ1A1. Co-expression of hGSTA1 with hCYP1A1 decreased DBP cytotoxicity 2-fold compared to V79MZh1A1 with hCYP1A1 alone, and provided a small, yet still statistically significant, 1.3-fold protection against DBPD. Protection against mutagenicity of these compounds was comparable to that for cytotoxicity in cells expressing hCYP1A1. In V79MZh1B1 cells, co-expression of hGSTA1 conferred up to 5-fold protection against DBP cytotoxicity, and up to 9-fold protection against the (+/−)-DBP-dihydrodiol cytotoxicity relative to the cells expressing hCYP1B1 alone. Co-expression of hGSTA1 also reduced mutagenicity of DBP or its dihydrodiol to a lesser extent (1.3-fold to 1.8-fold) than the protection against cytotoxicity in cells expressing hCYP1B1. These findings demonstrate that the protective efficacy of hGSTA1 against DBP and DBPD toxicity is variable, depending on the compound or metabolite present, the specific cytochrome P450 isozyme expressed, and the specific cellular damage endpoint examined.
Keywords: glutathione transferase, cytochrome P-450, polycyclic aromatic hydrocarbon, cytotoxicity, mutagenicity, transfection
II. Introduction
The class of environmental contaminants known as polycyclic aromatic hydrocarbons (PAH) are formed as a result of incomplete combustion of carbonaceous materials, and include numerous products that are potentially carcinogenic to humans [1]. The parent PAH procarcinogens are inactive but can be bioactivated to reactive electrophilic metabolites by cytochrome P450 (CYP) and other mixed function oxidases [2,3], or alternatively via one electron oxidation [4]. These reactive products include diol-epoxides with potent mutagenic and carcinogenic activity, some of which are substrates for conjugation by isozymes of the glutathione-S-transferase (GST) superfamily of genes [5]. Conjugation with the nucleophilic thiol group of the tripeptide glutathione (GSH) at the electrophilic centers of activated PAH renders their reactive groups such as epoxides less toxic, more water-soluble and hence more readily excretable [5,6]. Conjugation with GSH also flags toxic chemicals for export via plasma membrane efflux transporters, a step that is often required to complete the detoxification process [7,8]. Thus, GST expression is thought to play an important role in the prevention of the cytotoxicity, mutagenicity, and/or carcinogenicity resulting from PAHs in the environment [9–11]. The reactivity of the chemical species involved, and the balance between activation and subsequent detoxification and removal of reactive metabolites are critical factors that determine the toxicity and mutagenicity of PAHs in the cell.
Dibenzo[a,l]pyrene (DBP) is one of the most potent carcinogens known, with greater potency as a tumor initiator than other PAH more commonly studied such as dimethylbenzanthracene (DMBA) or benzo[a]pyrene (B[a]P) [12]. DBP is also a highly potent mutagen in both bacterial and mammalian cell in vitro mutagenicity assays [13,14]. Activation of DBP to the carcinogenic species is via initial oxidation to epoxides that are rapidly hydrolyzed to phenols or dihydrodiols, and subsequent further oxidation of the latter to highly reactive diol-epoxides. Activation of DBP proceeds through the intermediate production of DBP-11,12-dihydrodiol (DBP-diol) [14], and the most potent mutagenic metabolite appears to be dibenzo[a,l]pyrene-11,12-dihydrodiol-13,14-epoxide (DBPDE). Human cytochrome P450-1A1 (hCYP1A1) and cytochrome P450-1B1 (hCYP1B1), and to a lesser extent other CYPs, are known to catalyze the stereospecific biotransformation of DBP to yield predominantly two of the four possible stereoisomers, (+)-syn-DBPDE (S,R,S,R) and (−)-anti-DBPDE (R,S,S,R) [15,16]. The (−)-anti-DBPDE stereoisomer was responsible for the majority of DBPDE-DNA adducts, and was produced as a greater proportion of the total metabolites by hCYP1B1 as compared to hCYP1A1 [17,18]. Interestingly, however, activation via hCYP1A1-mediated metabolism of DBP elicited stronger cytotoxic effects [19], possibly due to polar DNA adducts of metabolites produced only in cells expressing hCYP1A1.
Studies of the conjugation of the most metabolically relevant (+)-syn and (−)-anti-DBPDE metabolites indicated that the hGSTP1-1 isozyme exhibited appreciable activity toward (−)-anti and (+)-syn enantiomers of DBP diol epoxides, with preference for conjugation of the (−)-anti-diastereoisomers [20,21]. More recent studies demonstrated that purified hGSTA1-1 exhibits at least an order of magnitude more efficient activity for the conjugation of (−)-anti-DBPDE compared to hGSTM1-1 and hGSTP1-1 [22]. The high carcinogenic potential of DBP metabolites and their efficacy as substrates for conjugation by GSTs suggests that one or more GST isozymes may serve an important function in their detoxification. Interestingly, however, the efficacy of DBPDE conjugation was reduced an order of magnitude when cytosol containing hGSTA1 was assayed instead of equivalent activities of the purified enzyme. Expression of hGSTA1 in cells reduced DNA adducts by 6.5-fold compared to the parent V79MZ cells, but since the highly reactive diol-epoxide must traverse the plasma membrane and reach the DNA in the nucleus, the normal intracellular compartmentation of DBPDE formation by CYP isozymes at the endoplasmic reticulum and its subsequent trafficking to intracellular targets are not accurately reproduced.
Until recently, studies on the toxicity of DBP and other putative human carcinogens have focused upon either the activation to reactive metabolites or their detoxification. However, relatively little focus has been placed upon understanding the dynamic competition when both activating and detoxifying enzymes are present in a cellular context in terms of relative sensitivity to toxic and mutagenic effects. We have previously demonstrated protection against carcinogens by expression of transfected GST genes, either alone when activation is not required, or in opposition to co-expressed CYP pathways when relevant to the usual route of activation of the carcinogen [23–27]. We have observed that the protective efficacy of GST often varies depending upon the biological endpoint examined, even with the same paired isogenic (CYP ± GST)-expressing cell lines. We have now extended these studies to examine the competing roles of CYP1A1 and CYP1B1 activation and hGSTA1-mediated protection in cell sensitivity to toxicity or mutagenicity of DBP and its metabolites, using stably transfected cell lines that are isogenic except for expression of either hCYP1A1 or hCYP1B1 alone, or each in concert with hGSTA1.
III. Materials and Methods
Materials and Chemicals
Caution: The PAH and PAH metabolites described herein are potential chemical carcinogens and must be handled with care as outlined in the National Cancer Institute Guidelines.
DMEM (Dulbecco’s Modified Eagle’s Medium) was purchased from Gibco (Grand Island, NY). Fetal bovine serum was purchased from Cellgro/Mediatech (Herndon, VA). Dibenzo[a,l]pyrene (CAS Registry No. 191-30-0) and racemic (+/−)-DBP-11,12-dihydrodiol were purchased from Midwest Research Institute (Lexena, KS). All other reagents were analytical grade and purchased from Fisher Scientific (Atlanta, GA) or Sigma (St. Louis, MO).
Cell Culture and Cell Lines
V79MZ cells transfected with human CYP1A1 or human CYP1B1 were developed by Dr. Johannes Doehmer and colleagues at the Technical Institute for Toxicology, Munich, Germany [18,28]. The human GSTA1 cDNA (generously provided by Dr. C-P. Tu, Penn State University) was ligated into the modified CMV promoter-driven ΔpCEP4 expression vector, and generation of the V79MZh1A1 or V79MZh1B1 cell lines stably expressing human glutathione-S-transferase A1 was by the method of calcium phosphate-mediated transfection followed by hygromycin selection, verification of similar growth rates, and GST analysis as described previously [26]. All cell lines were grown and maintained in DMEM medium supplemented with 5% FBS and 0.250 μg/ml G418 (Cellgro/Mediatech, Herndon, VA), at 37°C in 5% CO2. Cells were subcultured at 1:20 dilution every two to three days.
Enzyme Assays
The hCYP1A1 and hCYP1B1 enzyme activities in intact cells were measured using the fluorogenic substrate 7-benzyloxymethyloxy-3-cyanocoumarin (Vivid BOMCC; Invitrogen Corporation, Carlsbad, CA), which yields a blue 7-Hydroxycoumarin fluorescent product. This assay is much more sensitive than the ethoxyresorufin O-deethylase (EROD) assay, especially for detection of CYP1B1 activity. Transfected and control cells were plated in 60 mm cell culture dishes at 1.8 × 105 cells per plate in 5 mL DMEM and grown without selection for two days in a humidified atmosphere of 5% CO2, 95% air at 37°C. Plates were then rinsed twice with serum-free DMEM and covered with 2 mL of DMEM containing 5 μM Vivid BOMCC substrate and placed in a 37°C cell culture incubator for 30 min. At the end of the incubation period a one mL aliquot was taken from each plate and fluorescence was measured on a Perkin-Elmer model LS-3B spectrofluorimeter (Perkin-Elmer, Norwalk, CT) set for excitation at 409 nm and detection of emission at 460nm. Results were determined by comparison to a standard curve of the Vivid ® Blue Standard (Invitrogen Corporation, Carlsbad, CA) Activities were normalized for protein content as measured by the BCA assay, and was expressed as pmol/min/mg protein.
The method for GST assay is a modification of the method described by Habig [29] and has been previously described [27]. Cells were harvested, sonicated, and centrifuged as described previously, and protein concentration determined by the BCA assay. Cytosol (2–5 μl of supernatant, corresponding to 10–20 μg protein) was added to a solution of 0.1M K2PO4, pH 6.5, and 1 mM glutathione (GSH). The reaction was initiated with 1 mM (final concentration) 1-chloro-2,4-dinitrobenzene (CDNB) in 20X ethanolic solution. Change in absorbance was monitored at 340 nm for 90 seconds. Activity was calculated using the ΔA/minute and extinction coefficient, and was reported in nmol/min/mg protein.
Cytotoxicity Assays
Cells were plated at a density of 250 cells/well in 96 well plates, and allowed to attach and grow without G418 selection for 16–24 hours. Cells were exposed to DBP or (+/−)-DBP-11,12-dihydrodiol Page 7 (DBPD) continuously for 96 hours, harvested when control wells were at 90% confluence, and medium removed and cells fixed by addition of 100 μl cold 5% TCA for one hour at 4°C. Plates were rinsed under tap water, drained and allowed to dry, then stained with 0.4% sulforhodamine B (SRB) in 1% acetic acid for 10 minutes, rinsed in 1% acetic acid solution five times, and dried. The SRB was resolubilized in 0.1 M Tris base (pH 10) and plates read on a microplate spectrophotometer (Molecular Devices, Sunnyvale, CA) at 560 nm, as an indirect measure of cellular density via protein staining.
hprt Mutagenicity Assay
Cells were plated at a density of 5 × 105 cells per 100 mm plate in the absence of G418 selection, and allowed to adhere to the plates overnight. The indicated concentrations of DBP or (+/−)-DBPD were added to the cell culture medium (0.1% DMSO final concentration), and after a 48 hour exposure, medium containing PAH was removed, and 10 ml fresh medium was added and left on the cultures overnight. The next day, cells were then trypsinized, counted and replated for phenotypic development at 5 × 105 cells per plate and allowed to grow for 6 days with one subculture at three days. After this period, cells were plated at a density of 5 × 105 cells per 100 mm plate and subjected to cytotoxic selection with 6-thioguanine (10 μg/ml) to select for hprt mutants. After 10 days, mutant hprt colonies were fixed and stained with 3 ml of 0.16% methylene blue in methanol, rinsed with water, counted manually, and mutants expressed per million cells.
IV. Results
Transgenic Cell Lines
Analysis of hCYP1A1 activity in the V79MZh1A1 and hGSTA1-expressing derivative cell lines indicated a strong elevation of activity (110–113 pmol/min/mg) compared to V79MZ cells (0.33 pmol/min/mg) that have essentially no CYP expression. The activity of hCYP1B1 was 7.1 pmol/min/mg in the V79MZh1B1 cell line and 8.2 pmol/min/mg in the hGSTA1-expressing derivative line V79MZh1B1/hGSTA1-11. Although the specific activity of hCYP1A1 was higher than that of hCYP1B1 using the BOMCC substrate, the enzyme expression levels have been previously shown to be comparable in the V79MZh1A1 and V79MZh1B1 cell lines (18). The activity of hGSTA1-1 expressed in the double-transfected V79MZh1A1/hGSTA1-39 cells was 586 nmol/min/mg, and was 1101 ± 19 nmol/min/mg in V79MZh1B1/hGSTA1-11 cells (Table 1). Background GST activity was less than 200 nmol/min/mg in V79MZ, V79MZh1A1, and V79MZh1B1 cells, due to a low level expression of hamster GSTP1, which has previously been shown to be ineffective for the conjugation of benzo[a]pyrene diol epoxides in these cells [30]. Cultures were replaced with fresh cryopreserved stocks every 6–8 weeks, and monitored regularly to assure maintenance of CYP and GST activities.
Table 1. Enzymatic activities of CYP1A1 or CYP1B1 and GST in transgenic V79MZ Cell Lines.
Cell lines were plated, grown, harvested and assayed for CYP (using Vivid BOMCC substrate) or GST (CDNB substrate) specific activities as described in Materials and Methods. Protein was determined by the BCA method, and activity expressed as pmol/min/mg (CYP) or nmol/min/mg (GST).
| Cell Line | Genes Expressed | P450 Activity (pmol/min/mg) | GST (nmol/min/mg) |
|---|---|---|---|
| V79MZ (control) | no transgenes | 0.33 ± 0.21 | 173 ± 18 |
| V79MZh1A1 | hP4501A1 | 110 ± 12 | 175 ± 23 |
| V79MZh1A1/hGSTA-39 | hP4501A1 + hGSTA1-1 | 113 ± 24 | 586 ± 44 |
| V79MZh1B1 | hP4501B1 | 7.1 ± 1.0 | 128 ± 45 |
| V79MZh1B1/hGSTA-11 | hP4501B1 + hGSTA1-1 | 8.2 ± 1.5 | 1101 ± 19 |
Effect of hCYP1A1 or hCYP1B1 expression on DBP and DBP-11.12-dihydrodiol cytotoxicity
Cells were exposed to DBP or to the racemic mixture of (+/−)-DBP-11.12-dihydrodiol. Expression of the human CYP1A1 increased sensitivity to the parent compound by at least 40-fold, and to the DB[a]P-11.12-dihydrodiol metabolite by more than 140-fold, compared to the sensitivity of the unmodified V79MZ control cells (Table 2). Expression of the human CYP1B1 also increased sensitivity to both compounds, but less so than was observed with activation by hCYP1A1, with a minimum of 17- to 20-fold enhancement of cytotoxicity in comparison to the parent V79MZ cells (Table 2).
Table 2. Protection against DBP or (+/−)-11,12-DBP-dihydrodiol cytotoxicity by transfected hGSTA1 in transgenic V79 cells expressing hCYP1A1 or hCYP1B1.
Cytotoxicity assays were performed as described in Materials and Methods. Results are the mean +/− S.D. of 3 or more independent assays.
| Cell Line | DB[a,1]P IC50 (nM) | Fold-Resistance | (±)-DBP-11,12-Diol IC50 (nM) | Fold-Resistance |
|---|---|---|---|---|
| V79MZ (control) | > 100 | n/a | >100 | n/a |
| V79MZh1A1 | 2.4 ± 0.7a | (1.0) | 0.7 ± 0.2 | (1.0) |
| V79MZh1A1/hGSTA1-39 | 4.6 ± 1.7 | 1.9 | 0.9 ± 0.5b | 1.3 |
| V79MZh1B1 | 6.0 ± 1.0 | (1.0) | 4.8 ± 1.8 | (1.0) |
| V79MZh1B1/hGSTA1-11 | 31.6 ± 5.3c | 5.3 | 43.0 ± 9.0d | 9.0 |
significant difference from V79MZh1A1; p < 0.04
no significant difference from V79MZh1A1; p > 0.5
significant difference from V79MZh1B1; p < 0.003
significant difference from V79MZh1B1; p < 0.003
Effect of hGSTA1 expression on DBP and DBP-11.12-dihydrodiol cytotoxicity
Co-expression of human GSTA1 in the V79MZh1A1/hGSTA1-39 cell line conferred a modest 1.9-fold resistance to DBP-mediated cytotoxicity (IC50 = 4.6 nM compared to 2.4 nM for V79MZh1A1, p < 0.04) (Table 2). However, the IC50 values were not significantly different for the (+/−)-DBP-11,12-dihydrodiol between the V79MZh1A1 and its hGSTA1-expressing derivative line (IC50 = 0.7 nM for V79MZh1A1, vs. IC50 = 0.9 nM for V79MZh1A1/hGSTA1-39; p > 0.5).
Co-expression of hGSTA1 conferred greater fold-resistance to the cytotoxicity of either DBP or DBP-11,12-dihydrodiol in cells expressing CYP1B1. Compared to the V79MZh1B1 cells, a 5.3-fold resistance to DBP cytotoxicity was observed in V79MZh1B1/GSTA-11 cells (IC50 = 31.6 nM compared to 6.0 nM for V79MZh1B1; p < 0.003), and 9.0-fold protection was conferred against the DBP-11,12-dihydrodiol (IC50 = 43.0 nM compared to 4.8 nM for V79MZh1B1; p < 0.003).
Effect of hCYP1A1 or hCYP1B1 expression on DBP and DBP-11.12-dihydrodiol mutagenicity
Mutagenicity was measured by the frequency of mutation at the hprt locus following exposure of the cells to DBP or the DBP-11.12-diol. Dose-dependent mutagenicity was observed in the cell lines expressing hCYP1A1 or hCYP1B1 but was negligible in the parental V79MZ cells, which lack CYP activity (Figures 1 and 2). In contrast to the greater cytotoxicity in the V79hCYP1A1 cell line, the cells expressing hCYP1B1 were 3-fold more sensitive to mutagenesis of DBP, or 2-fold more sensitive to mutagenesis of (+/−)-DBP-11,12-diol in comparison with cells expressing hCYP1A1, across all concentrations tested (Figures 1 and 2, p < 0.04).
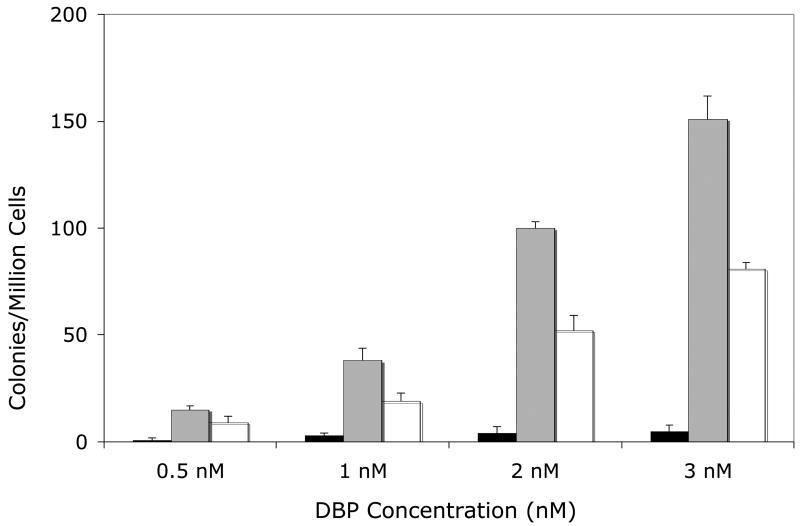
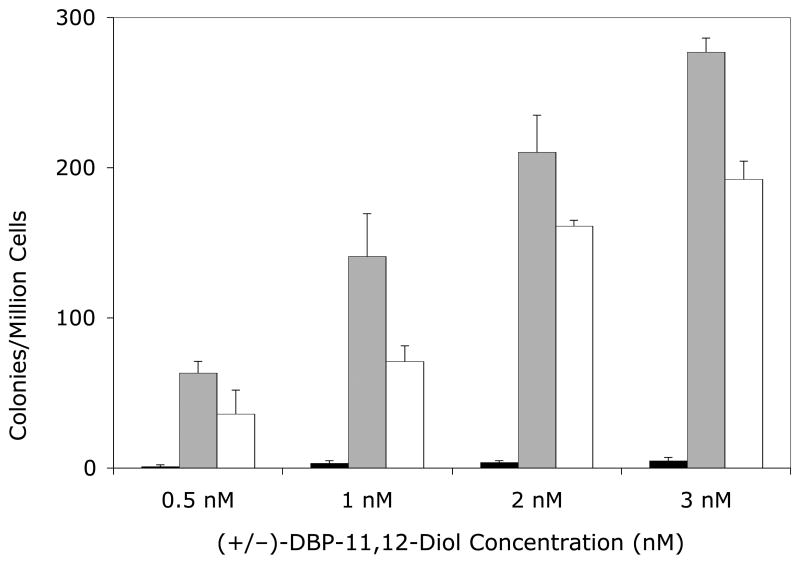
Figure 1. Mutagenicity of DBP (Panel A) or (±)-DBP-11.12-dihydrodiol (Panel B) in the V79MZh1A1 cell line, with or without transfected hGSTA1-1.
Solid bars, V79MZ control cells; gray bars, V79MZh1A1 cells (expressing hCYP1A1); open bars, V79MZh1A1/hGSTA1-39 (expressing both hCYP1A1 and hGSTA1). Results are the mean ± S.D. of at least three independent experiments.
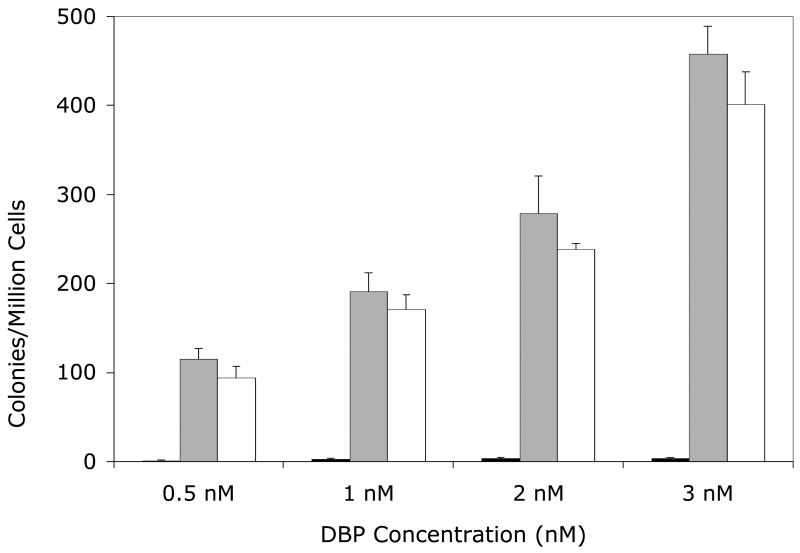
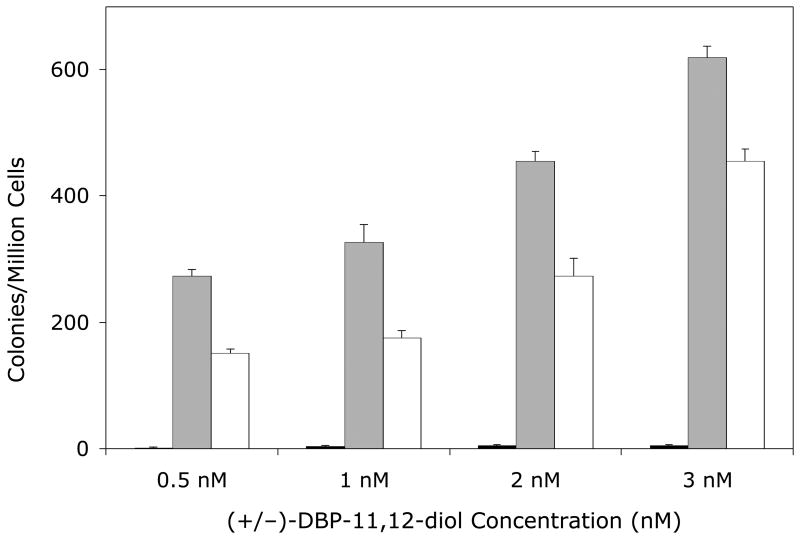
Figure 2. Mutagenicity of DBP (Panel A) or (±)-DBP-11.12-dihydrodiol (Panel B) in the V79MZh1B1 cell line, with or without transfected hGSTA1-1.
Solid bars, V79MZ control cells; gray bars, V79MZh1B1 cells (expressing hCYP1B1); open bars, V79MZh1B1/hGSTA1-11 (expressing both hCYP1A1 and hGSTA1). Results are the mean ± S.D. of at least three independent experiments.
GST Protection Against Mutagenicity of Dibenzo[a,l]pyrene or (+/−)-DB[a]P-11.12-Dihydrodiol
Co-expression of hGSTA1 reduced the mutagenicity of DBP by about 50% in cells expressing hCYP1A1 (Figure 1A, p < 0.04), and reduced the mutagenicity of the (+/−)-DBP-11,12-diol by an average of 37% (range = 25 – 50% reduction, Figure 1B, p < 0.04). Somewhat lower protection was conferred by hGSTA1 against DBP in V79MZh1B1/hGSTA1-11 cells, with an average reduction of 29% (range = 20 – 43%, Figure 3A, p < .04). Mutagenicity of the (+/−)-DBP-11, 12-dihydrodiol was reduced by 26 – 46% in V79MZh1B1 + hGSTA1-11 cells compared to V79MZ1B1 (Figure 2B, p < 0.009). Overall, the presence of hGSTA1 expression confers a modest, but statistically significant reduction in the genotoxicity of the products of the activation of DBP or (±)-DBP-11,12-diol by hCYP1A1 or hCYP1B1.
V. Discussion
A number of studies have examined metabolic activation, by various purified or expressed cytochrome P450 isozymes, of polycyclic aromatic hydrocarbons via oxidation to reactive species that are potentially cytotoxic and genotoxic. Cellular sensitivity to toxicity or mutagenicity was shown to vary with the particular P450 isozyme expressed and the PAH undergoing metabolism. Similarly, expression of transfected GST isozymes has been shown to protect against the cytotoxicity or mutagenicity of the ultimate PAH diol-epoxide metabolites added to cell culture medium. However, relatively little is known about the protective efficacy of GST in direct competition with ongoing activation by CYP expressed together in the same cell. The V79 cell lines co-expressing a human cytochrome P450 and a human GST allow investigation of the relative protection of GST in the context of the intracellular dynamics of activation versus detoxification rates, and in the presence of the complex range of metabolites generated via CYP oxidation of the PAH. This novel approach to understanding the role of GSTs in the prevention of toxic and mutagenic damage will help fill a critical gap in the understanding of xenobiotic metabolism and implications for toxicity.
Since the hGSTA1 isozyme has been shown to have the highest activity for conjugation of DBPDE among the human GSTs tested [22], we chose to focus initially on whether expression of hGSTA1 was effective for protection against cytotoxicity and mutagenicity of reactive CYP metabolites of the polycyclic aromatic hydrocarbon dibenzo[a,l]pyrene or its (+/−)-11,12-dihydrodiol intermediate metabolite. Both human CYP1A1 and CYP1B1 catalyze the activation of DBP to reactive metabolites that bind to cellular macromolecules [18,19]; therefore we developed transgenic model cell lines with either hCYP1A1 or hCYP1B1 expression, either alone or with co-expressed hGSTA1, for use in determining the efficacy of GST protection.
As expected, both hCYP1A1 and hCYP1B1 activated DBP and (+/−)-DBP-11,12-dihydrodiol to more toxic and more mutagenic metabolites. The several-fold greater potency of both compounds in the cytotoxicity assay with the hCYP1A1-expressing line than in the hCYP1B1-expressing line is consistent with results of an earlier study with these cell lines [19]. The greater cytotoxicity with hCYP1A1 activation may be due to polar metabolites of DBP other than DBPDE that were observed in a previous study with the V79MZhCYP1A1 cells, but not with the V79MZhCYP1B1 cells [18]. The (+/−) DB-11,12-dihydrodiol is substantially more potent in the cytotoxicity assay than the parent compound DBP with the V79hCYP1A1 cells, whereas both compounds have similar cytotoxicity in the V79MZh1B1 cells as indicated by the similar IC50 values for each compound. Assuming that formation of toxic activated metabolites occurs in a series of sequential stepwise oxidations as with benzo[a]pyrene [19], the greater potency of the (+/−)-DB-11,12-dihydrodiol in the V79hCYP1A1 cells suggests that the rate of formation of (+/−)-DB-11,12-dihydrodiol via oxidation of DBP is rate-limiting for generation of the ultimate cytotoxic species, presumably DBPDE. In contrast, both the parent compound and the dihydrodiol have similar cytotoxicity, and higher IC50 values in the cells expressing hCYP1B1, indicating that the dihydrodiol is not more efficiently activated than the parent compound by hCYP1B1, as was observed with the hCYP1A1-expressing cells.
Results showed that the fold-protection conferred by the hGSTA1 against DBP cytotoxicity was greater in the cells expressing hCYP1B1 and hGSTA1 than for those expressing hCYP1A1 and hGSTA1 (5.3-fold vs. 1.9-fold, respectively). This might be largely explained on the basis of the higher expression of hGSTA1 in the V79MZhCYP1A1/hGSTA1B1-11 cell line (1101 vs. 586 mU/mg in the V79MZh1A1/hGSTA1-39 line, respectively). However, in view of the even greater parallel difference in protection against the DBP-11,12-dihydrodiol cytotoxicity (9.0-fold vs. 1.3-fold, respectively), we must also consider the possibility that at least some of the metabolites formed from CYP1A1-mediated activation of these compounds may not be as efficiently detoxified by the hGSTA1 as species formed via CYP1B1-mediated metabolism. Indeed, as mentioned previously, an unidentified subset of polar DNA adducts was observed in the DBP-treated V79MZh1A1 cells that was absent in V79MZh1B1 cells exposed to DBP or DBP-11,12-dihydrodiol [31]. In addition to possibly being weaker substrates for detoxification by hGSTA1 conjugation, these metabolites may also be more toxic than the (−)-anti-DBPDE that is the principal activated metabolite formed via hCYP1B1, as suggested by the lower IC50 values in the cytotoxicity assay with the hCYP1A1 cells for both DBP and the dihydrodiol.
In contrast to the cytotoxicity results, the sensitivity to mutagenicity was substantially greater for both compounds in the V79MZhCYP1B1 cell line, as compared to the V79MZhCYP1A1 cell line. However, co-expression of hGSTA1 conferred 2-fold or less reduction in the mutagenicity of DBP or DB-11,12-dihydrodiol in the cells expressing either hCYP1A1 or hCYP1B1. This was unexpected in the case of activation by hCYP1B1, as hGSTA1 had provided 5.3-fold and 9-fold protection against cytotoxicity of DBP or the dihydrodiol, respectively in these cells. We have observed a similar discrepancy in other studies with B[a]P and B[a]P-7,8-diols in V79 cells co-expressing hCYP1A1 and hGSTP1-1, in which protection against cytotoxicity was much greater than protection against genotoxicity [23,32] (and unpublished observations). The disparate results for the different endpoints with activation of B[a]P, DBP or their dihydrodiols by CYP isozymes expressed along with GST in the target cells are in contrast to our earlier finding that transgenic expression of hGSTP1 conferred a similar 5-fold protection against both cytotoxicity and DNA adduct formation induced by the activated metabolite BPDE in T47D breast cancer cells [25]. Thus, the different degree of protection by GSTs against different end-points appears to be related to the activation in situ by CYP isozymes, suggesting that different metabolites or stereoisomers may mediate the cytotoxic and genotoxic effects, and these may be detoxified by GSTs to different extents. Furthermore, studies by others have indicated that partitioning of these hydrophobic metabolites in membrane compartments of the cell may limit the amount of available substrate for the GST [22]. Overall, these results suggest several possible explanations: 1) multiple different metabolites may mediate the cytotoxic versus genotoxic effects of these PAH; 2) key target(s) other than, or in addition to DNA are likely involved in the cytotoxic effects; and 3) the key targets and/or metabolites may be compartmentalized in a manner that affects the ability of GST to intervene between the site of activation and the respective targets for the different endpoints. The latter point, if correct, would help to explain our observation that protection by GST against PAH activated in situ in cells expressing both CYP and GST is generally more effective against the cytotoxic than genotoxic effects of the PAH metabolites generated. These potential mechanisms are currently under investigation.
Acknowledgments
This work was supported by a grant from the National Institute for Environmental Health Sciences, NIH, # RO1-ES-10175, and by training grant # T32-ES-007331 (M.E.K. and S.A).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes and Control. 1997;8:444–472. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- 2.Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and1B1. Cancer Science. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sims P, Grover PL, Swaisland A, Pal K, Hewer A. Metabolic activation of benzo(a)pyrene proceeds by a diol-epoxide. Nature. 1974;252:326–328. doi: 10.1038/252326a0. [DOI] [PubMed] [Google Scholar]
- 4.Cavalieri E, Rogan E. Central role of radical cations in metabolic activation of polycyclic aromatic hydrocarbons. Xenobiotica. 1995;25:677–688. doi: 10.3109/00498259509061885. [DOI] [PubMed] [Google Scholar]
- 5.Hayes JD, Pulford DJ. The glutathione S-Transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 6.Hayes JD, Flanagan JU, Jowsey IR. Glutathione Transferases. Annual Review of Pharmacology and Toxicology. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends in Biochemical Sciences. 1992;17:463–468. doi: 10.1016/0968-0004(92)90489-v. [see comments] [DOI] [PubMed] [Google Scholar]
- 8.Morrow CS, Smitherman PK, Diah SK, Schneider E, Townsend AJ. Coordinated action of glutathione S-transferases (GST) and multidrug resistance protein (MRP) in antineoplastic drug detoxification : Mechanism of GSTA1-1– and MRP–associated resistance to chlorambucil in MCF-7 breast carcinoma cells. J Biol Chem. 1998;273:20114–20120. doi: 10.1074/jbc.273.32.20114. [DOI] [PubMed] [Google Scholar]
- 9.Talalay P, Dinkova-Kostova AT, Holtzclaw WD. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Advances in Enzyme Regulation. 2003;43:121–134. doi: 10.1016/s0065-2571(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 10.Mannervik B. Evolution of glutathione transferases and related enzymes for the protection of cells against electrophiles. Biochem Soc Trans. 1996;24:878–880. doi: 10.1042/bst0240878. [DOI] [PubMed] [Google Scholar]
- 11.Ketterer B. Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. Mutation Research. 1988;202:343–361. doi: 10.1016/0027-5107(88)90197-2. [Review] [DOI] [PubMed] [Google Scholar]
- 12.Higginbotham S, RamaKrishna NVS, Johansson SL, Rogan EG, Cavalieri EL. Tumor-initiating activity and carcinogenicity of dibenzo[a,l]pyrene versus 7, 12-dimethylbenz[a]anthracene and benzo[a]pyrene at low doses in mouse skin. Carcinogenesis. 1993;14:875–878. doi: 10.1093/carcin/14.5.875. [DOI] [PubMed] [Google Scholar]
- 13.Busby W, Smith H, Crespi C, Penman B. Mutagenicity of benzo[a]pyrene and dibenzopyrenes in the Salmonella typhimurium TM677 and the MCL-5 human cell forward mutation assays. Mutat Res. 1995;342:9–16. doi: 10.1016/0165-1218(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 14.Luch A, Seidel A, Glatt H, Platt KL. Metabolic Activation of the (+)-(S,S)- and (−)-(R,R)-Enantiomers of (trans)-11,12-Dihydroxy-11,12-dihydrodibenzo[a,l]pyrene: Stereoselectivity, DNA Adduct Formation, and Mutagenicity in Chinese Hamster V79 Cells. Chem Res Toxicol. 1997;10:1161–1170. doi: 10.1021/tx970005i. [DOI] [PubMed] [Google Scholar]
- 15.Shou M, Krausz K, Gonzalez F, Gelboin H. Metabolic activation of the potent carcinogen dibenzo[a,l]pyrene by human recombinant cytochromes P450, lung and liver microsomes. Carcinogenesis. 1996;17:2429–2433. doi: 10.1093/carcin/17.11.2429. [DOI] [PubMed] [Google Scholar]
- 16.Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Research. 1996;56:2979–2984. [PubMed] [Google Scholar]
- 17.Ralston SL, Coffing SL, Seidel A, Luch A, Platt KL, Baird WM. Stereoselective Activation of Dibenzo[a,l]pyrene and Its (trans)-11,12-Dihydrodiol to Fjord Region 11,12-Diol 13,14-Epoxides in a Human Mammary Carcinoma MCF-7 Cell-Mediated V79 Cell Mutation Assay. Chem Res Toxicol. 1997;10:687–693. doi: 10.1021/tx9700275. [DOI] [PubMed] [Google Scholar]
- 18.Luch A, Coffing SL, Tang YM, Schneider A, Soballa V, Greim H, Jefcoate CR, Seidel A, Greenlee WF, Baird WM, Doehmer J. Stable expression of human cytochrome P450 1B1 in V79 Chinese hamster cells and metabolically catalyzed DNA adduct formation of dibenzo[a,l]pyrene. Chemical Research in Toxicology. 1998;11:686–695. doi: 10.1021/tx970236p. [DOI] [PubMed] [Google Scholar]
- 19.Luch A, Schober W, Soballa VJ, Raab G, Greim H, Jacob J, Doehmer J, Seidel A. Metabolic activation of dibenzo[a,l]pyrene by human cytochrome P450 1A1 and P450 1B1 expressed in V79 Chinese hamster cells. Chem Res Toxicol. 1999;12:353–364. doi: 10.1021/tx980240g. [DOI] [PubMed] [Google Scholar]
- 20.Sundberg K, Widersten M, Seidel A, Mannervik B, Jernström B. Glutathione conjugation of bay- and fjord-region diol epoxides of polycyclic aromatic hydrocarbons by glutathione transferases M1-1 and P1-1. Chem Res Toxicol. 1997;10:1221–1227. doi: 10.1021/tx970099w. [DOI] [PubMed] [Google Scholar]
- 21.Sundberg K, Johansson AS, Stenberg G, Widersten M, Seidel A, Mannervik B, Jernström B. Differences in the catalytic efficiencies of allelic variants of glutathione transferase P1-1 towards carcinogenic diol epoxides of polycyclic aromatic hydrocarbons. Carcinogenesis. 1998;19:433–436. doi: 10.1093/carcin/19.3.433. [DOI] [PubMed] [Google Scholar]
- 22.Sundberg K, Dreij K, Seidel A, Jernstrom B. Glutathione Conjugation and DNA Adduct Formation of Dibenzo[a,l]pyrene and Benzo[a]pyrene Diol Epoxides in V79 Cells Stably Expressing Different Human Glutathione Transferases. Chem Res Toxicol. 2002;15:170–179. doi: 10.1021/tx015546t. [DOI] [PubMed] [Google Scholar]
- 23.Townsend AJ, Kabler SL, Doehmer J, Morrow CS. Modeling the metabolic competency of glutathione S-transferases using genetically modified cell lines. Toxicol. 2002;181–182:265–269. doi: 10.1016/s0300-483x(02)00294-9. [DOI] [PubMed] [Google Scholar]
- 24.Townsend AJ, Fields WR, Doss AJ, Clapper ML, Doehmer J, Morrow CS. Modeling the Chemoprotective Functions of Glutathione S-Transferases in Cultured Cell Lines by Heterologous Expression. Drug Metab Rev. 1998;31:43 – 69. doi: 10.1081/dmr-100101907. [DOI] [PubMed] [Google Scholar]
- 25.Fields WR, Morrow CS, Doss AJ, Sundberg K, Jernström B, Townsend AJ. Overexpression of stably transfected human glutathione S-Transferase p1-1 protects against DNA damage by benzo[a]pyrene diol-epoxide in human T47D cells. Mol Pharmacol. 1998;54:298–304. doi: 10.1124/mol.54.2.298. [DOI] [PubMed] [Google Scholar]
- 26.Townsend AJ, Fields WR, Haynes RL, Karper AJ, Li Y, Doehmer J, Morrow CS. Chemoprotective functions of glutathione s-transferases in cell lines induced to express specific isozymes by stable transfection. Chem Biol Interact. 1998;111–112:389–407. doi: 10.1016/s0009-2797(97)00175-0. [DOI] [PubMed] [Google Scholar]
- 27.Fields WR, Li Y, Townsend AJ. Protection by transfected glutathione S-transferase isozymes against carcinogen-induced alkylation of cellular macromolecules in human MCF-7 cells. Carcinogenesis. 1994;15:1155–1160. doi: 10.1093/carcin/15.6.1155. [DOI] [PubMed] [Google Scholar]
- 28.Schmalix WA, Maser H, Kiefer F, Reen R, Wiebel FJ, Gonzalez F, Seidel A, Glatt H, Greim H, Doehmer J. Stable expression of human cytochrome P450 1A1 cDNA in V79 Chinese hamster cells and metabolic activation of benzo[a]pyrene. European Journal of Pharmacology. 1993;248:251–261. doi: 10.1016/0926-6917(93)90052-r. [DOI] [PubMed] [Google Scholar]
- 29.Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods In Enzymology. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 30.Swedmark S, Romert L, Morgenstern R, Jenssen D. Studies on glutathione transferases belonging to class pi in cell lines with different capacities for conjugating (+)-7beta,8alpha-dihydroxy-9alpha,10alpha-oxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Carcinogenesis. 1992;13:1719–1723. doi: 10.1093/carcin/13.10.1719. [DOI] [PubMed] [Google Scholar]
- 31.Luch A, Schober W, Soballa VJ, Raab G, Greim H, Jacob J, Doehmer J, Seidel A. Metabolic Activation of Dibenzo[a,l]pyrene by Human Cytochrome P450 1A1 and P450 1B1 Expressed in V79 Chinese Hamster Cells. Chem Res Toxicol. 1999;12:353–364. doi: 10.1021/tx980240g. [DOI] [PubMed] [Google Scholar]
- 32.Kushman ME, Kabler SL, Fleming MH, Morrow CS, Townsend AJ. Expression of hGSTP1 confers resistance to benzo[a]pyrene mutagenesis in stably transfected V79MZ cells co-expressing hCYP1A1. Carcinogenesis. 2007;28:207–214. doi: 10.1093/carcin/bgl125. [DOI] [PubMed] [Google Scholar]