Abstract
Accurate detection of the earliest signs of ischemia on the surface electrocardiogram (ECG) is essential for timely diagnosis and management of potentially life-threatening ischemic events. Yet, accuracy of ischemia analysis in ECG monitors remains suboptimal due to a number of confounding factors, including changes in body position and other artifacts. Hence, the goals of this study were 1) to examine the duration and time course of ischemic events and 2) to compare ECG changes caused by “true” ischemic events with those caused by changes in body position. Continuous, 12-lead Holter ECGs obtained from patients who presented to the Emergency Department (ED) with chest pain and enrolled in the IMMEDIATE AIM Study were analyzed. Holter recordings were initiated within the 1st 40 min after patients' arrival to the ED. Here we present preliminary results.
Methods
Twelve patients (age:59±16, 5 female, 2 with a final diagnosis of non-STEMI, 4 with unstable angina, and 6 with other cardiovascular disease) in whom ischemic ST-deviations were identified on Holter data, underwent four consecutive, 2-min recordings in the following body positions: 1) supine, 2) on the left side, 3) on the right side, and 4) sitting (or standing) upright. After baseline correction, beat-to-beat changes in QRS and STT-segments were examined in all 8 channels and the root-mean-square (RMS)-curve using an adaptive algorithm that computes the slope, amplitude, duration, area, and the Karhunen-Loeve derived representation of the corresponding segment. To prevent possible biases towards patients with more frequent ischemic events, a single, index event was chosen for analysis in each patient. There were 3 ST-elevation events and 9 ST-depression events; these events reached the maximum ST-deviation 11±8 hours (mean ± standard deviation) after the beginning of the recording.
Results and Conclusions
In most patients with transient myocardial ischemia, the microvolt-level, sub-threshold deviation of the ST-segment developed gradually, over 15-20 min, until it reached the maximum, super-threshold level. Despite the different ischemia localizations, the RMS-curve allowed accurate detection of significant changes in the ST-segment in the studied group (Friedman ANOVA for repeated measurements over 1-hour interval). Changes in body position could be identified by tracking dynamics of the QRS pattern/axis. Adaptive algorithms for tracking of the ST-dynamics with simultaneous tracking of the patterns of QRS-complexes to discriminate the “true” and “false”-positive events are presented and discussed.
Keywords: Ischemia, Electrocardiography, Monitoring
Introduction
Accurate detection of the earliest signs of ischemia on the surface electrocardiogram (ECG) is essential for timely diagnosis and management of potentially life-threatening ischemic events. Yet, the accuracy of ischemia analysis in ECG monitors remains suboptimal due to a number of confounding factors, including changes in body position and other artifacts. Hence, the goals of this study were 1) to examine the duration and time course of ischemic events and 2) to compare ECG changes caused by “true” ischemic events with those caused by changes in body position.
Variation in the magnitude of ST-segment amplitude due to changes in body position, herein referred to as positional ECG change, is a long-standing problem in clinical ECG monitoring and, in particular, monitoring of patients in the Emergency Room (ER) and other in-hospital units. Such positional ECG changes generate a number of false alarms prompting medical personnel to switch the ST-segment monitoring option off, which leaves the patients without ischemia monitoring. As a result, “true” ischemic events are often missed, misdiagnosed, or diagnosed with a significant time delay. Therefore, more accurate analytical tools and software are urgently needed to improve the accuracy of ischemia monitoring.
The problem of positional false-alarms has been addressed in several studies, and a number of analytical approaches for solving this problem have been suggested. These include: 1) recording of ECG patterns (templates) in each position and then using those templates as a reference to determine whether a change in body position had occurred,1,2,3 2) analyzing the time course of changes in the ST-segment while taking into account the slow, gradual time course of development of ischemia as opposed to more abrupt positional changes,4 3) taking into account the presence of high-frequency noise as an indicator of movements associated with positional changes,5 4) analyzing the duration of time spent in a particular body position while observing that the likelihood of more than one change in body position within <10 sec is small,5 and 5) making the algorithms adaptive to slow changes in QRS morphology to improve the detection of “true”, abrupt positional changes.5 Yet, despite the advances in signal processing and the improved understanding of the differences between positional and ischemic ECG changes, the problem of accurate ischemia monitoring with reliable suppression of false alarms remains unsolved.
One limitation of previous studies was related to the lack of ECG data with clearly annotated positional changes and ischemic events obtained in the same patient population during the same 24-hour period. To obviate this problem, previous investigations used separate datasets obtained from different groups of patients for the ischemia detection and analysis of positional changes, which made the comparison and interpretation of the results difficult. To the best of our knowledge, our study is the first to present the analysis of Holter ECG data obtained in a group of continuously monitored patients who had both ischemic and positional events (annotated) during the same 24-hour period. Here we show that the ischemic changes of the ST-segment develop gradually reaching super-threshold level after 15-20 min of sub-threshold ischemia, and describe the adaptive, multiparametric algorithms which have the potential to improve the accuracy of ischemia monitoring.
Methods
A. Sample
In this preliminary study, we examined continuous 12-lead Holter data obtained from patients who presented to the Emergency Department (ED) with chest pain and enrolled in the IMMEDIATE AIM Study (Ischemia Monitoring and Mapping in the Emergency Department In Appropriate Triage and Evaluation of Acute Ischemic Myocardium). Standard (Mason-Likar), 12-lead Holter ECG recordings were initiated within the 1st 40 min after patients' arrival to the ED. For the analysis, we selected patients in whom spontaneous ischemic ST-events were diagnosed by a cardiologist (KEF) and who underwent four consecutive, 2-min recordings in the following body positions (herein referred to as the positional tests or positional changes): 1) supine, 2) on the left side, 3) on the right side, and 4) sitting (or standing) upright. There were 12 patients (age: 59±16 years, 5 female, 2 with a final diagnosis of non-STEMI, 4 with unstable angina, and 6 with other cardiovascular diseases) matching these criteria. To prevent possible biases towards patients with more frequent ischemic events, a single, index ischemic event was chosen for analysis in each patient.
B. ECG processing in previous studies
a. Preprocessing
ECG preprocessing includes correction of baseline wander, detection, and classification of QRS-complexes. Several baseline correction algorithms have been developed, including the application of cubic spline, high-pass filtering, and adaptive approaches.6 Yet, application of these algorithms still leaves some residual baseline wander which might affect the accuracy of analysis of subtle changes in the amplitude of ST-segment and T-wave morphology.6 If the analysis is performed at the level of ultra-small beat-to-beat variations, an additional estimation of baseline stability is required to ensure proper separation of baseline artifact.7
b. Processing: Methods for continuous analysis of ST-amplitude
Three approaches for tracking the dynamics of the amplitude of the ST-segment have been proposed: 1) the spatial approach based on vectorcardiographic loop rotation angles, 2) the scalar approach based on the Karhunen–Loève transform (KLT) coefficients,5,8 and 3) the RMS difference series.9 Several studies investigated these approaches and found their performance comparable.5,8,9 The accuracy reported in several datasets was sufficiently high (>90%), but the false alarms could not be completely suppressed. Adaptive procedures have also been proposed to account for low-frequency drifts and high-frequency noises. For example, Jager et al. introduced an adaptive procedure using KLT that classifies changes in the ST-amplitude as ischemic or non-ischemic depending on the presence of QRS-axis shift with adaptation of the decision thresholds to slow drifts.10
c. Post-processing
At the post-processing phase, most studies utilize some type of filtering of high-frequency noise, using adaptive thresholds.5,10 In particular, adaptive, low-pass filtering of the KLT time series, filtering of the outliers using a median filter, and Bayesian detector have been proposed.5 The presence of short-term, high-frequency noise has also been used as an additional indicator of a transition in body position.5 The minimum time window of 10 sec for each positional change has been used to reject the short-term, high-frequency artifacts, due to an observation that the likelihood of >1 change in body position within 10 sec in patients on bed-side monitoring is relatively small.5 However, this empirically selected time window could be problematic in restless patients with chest pain, shock, anxiety, or other symptoms.
C. Processing in present study using a hidden-Markov-type neural network
We have extended previously used adaptive procedures by developing a neural network based on a modified hidden Markov model for processing a set of parameters referred herein to as the primary elements (50 elements in each channel and on the root-mean-square (RMS) curve) that completely describe the ECG waveform patterns.11,12 Using the transition and emission probability matrices, this network provides an infrastructure for monitoring and tracking of the system dynamics, including interactions between the primary elements.13
After baseline correction,6 beat-to-beat changes in the P, QRS and STT-segments were examined in all channels and on the RMS-curve using this set of primary elements that included the slope, amplitude, interval, duration, area, and the KLT- derived representation of the corresponding segment.7,14 The algorithm then learns and tracks dynamically the changes in these elements. If several types (patterns) of QRS or STT-segments are present in the same patient, the algorithm tracks the dynamics of each pattern (template) separately and uses cumulative template-averaging to adapt to slow changes in each pattern. The ischemic changes are identified using the modified hidden Markov model with two transition-probability matrices. One matrix determines the transition probabilities based on the group data; the second matrix performs individual adjustment (tailoring) of the probabilities in the first matrix to the individual patient.11,12,13 To minimize the effects of different data distributions, a nonparametric Friedman ANOVA for repeated measures was used to identify significant changes in the time series of the corresponding primary elements. P≤0.05 was considered statistically significant. The results are presented as mean ± standard deviation unless indicated otherwise.
Results
Positional changes in the ECG recordings
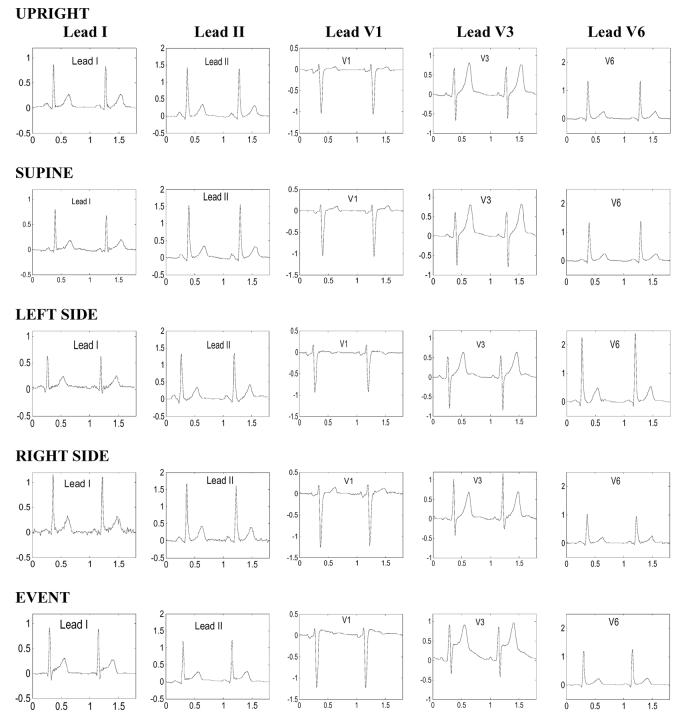
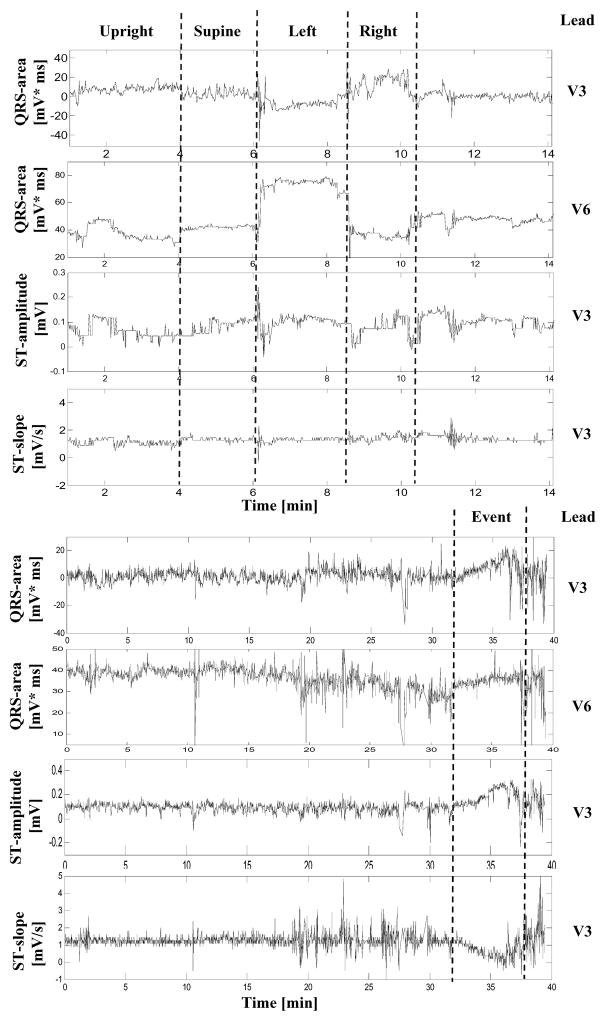
Figure 1 shows an example of ECGs recorded in a 46-year-old male patient during the positional tests (rows 1-4). The time series of several primary elements derived from consecutive cardiac complexes during each positional test in the same patient are shown in Figure 2, including QRS-areas in leads V3 and V6, and the ST-segment amplitude and slope in lead V3. Note reciprocal changes in the magnitude of the QRS complex during positional changes in leads V1 and V6 (Figure 1). As expected, when the patient is lying on the left side, the magnitude of the QRS complex increases in V6 and decreases in V1, whereas on the right side, the changes in QRS-area have an opposite direction (Figure 1). Also note that changes in the QRS-area are abrupt, clearly marking the transitions between different positions (Figure 2). However, changes in the amplitude of the ST segment are less obvious, although the high-frequency artifacts during the body rotation are clearly visible in the tracing. During the noise-free periods, when the patient remains in the same body position, deviations in the amplitude of the ST-segment are limited to < 100 μV.
Figure 1.
Electrocardiographic waveforms in leads I, II, V1, V3, and V6 (columns 1-5, respectively) obtained from a 46-year old male with transient chest pain thought to be due to coronary vasospasm during positional tests (rows 1-4) and an ischemic event (row 5).
Figure 2.
Electrocardiographic changes during positional tests (Row 1-4) and cardiac ischemia (Row 5-8) in the patient whose ECG waveforms are shown in Figure 1: QRS area in lead V3 (1st row), QRS area in lead V6 (2nd row), ST-amplitude in lead V3 (3rd row), ST-slope in lead V3 (4th row). ECG data in the same channels during an ischemic event are shown in rows 4-8. The time series ends at the time of maximum ST-elevation.
Figure 3 shows positional ECG changes (time series of the QRS-area in leads V3 and V6, and the ST-amplitude and slope in V6) in a 74-year old female with transient ischemic events. The magnitude of QRS-area in V3 decreases when the patient is on the right side and increases when the patient is on the left side. However, in contrast to the reciprocal pattern of changes in V3 and V6 shown in Figure 2, changes in the QRS-area in these two leads are non-reciprocal (i.e. both QRS-areas increase when the patient is on the left side). These inter-individual differences are most likely due to the different geometric positions of the cardiac vectors in the two patients. Note also that during noise-free periods of stable body positions, variations in the amplitude of the ST-segment in this patient are also within the 100-μV range.
Figure 3.
Electrocardiographic changes during positional tests (Row 1-4) and cardiac ischemia (Row 5-8) in a 74-year old female with transient ischemic events: QRS area in lead V3 (1st row), QRS area in lead V6 (2nd row), ST-amplitude in lead V6 (3rd row), ST-slope in lead V6 (4th row). ECG data in the same channels during an ischemic event is shown in rows 4-8. Note that ST-segment depression in lead V6 was sub-threshold for approximately 15 min (<.1mV), before becoming super-threshold during the last 5 min (>.1mV). The time series ends at the time of maximum ST-depression.
Electrocardiographic changes during ischemic events
Overall, patients in the studied group had 3 ST-elevation events and 9 ST-depression events; these reached the maximum ST-deviation 11±8 hours after the beginning of the recording. Figure 1, bottom row, shows changes in the ECG waveforms during an ischemic event that occurred within the 24-hour period of monitoring. Figure 2, lower half, shows continuous tracings recorded during the hour before the event. Note the gradual pattern of changes in all tracings that is clearly different from the abrupt changes during positional tests in the upper tracings. Comparing the ECG waveforms obtained during the event (Figure 1, bottom row) with those during different positional tests (Figure 1, upper rows), one can observe that the waveforms during the event were similar to those recorded in the supine and upright positions (Figure 1, first and second rows), suggesting that the patient was in one of those positions during the event. However, there are subtle differences in the magnitude of ECG waveforms. In particular, the magnitude of the S-wave is smaller during the event compared to that in the supine position. The higher magnitude of the J-point and the smaller S-wave during the event lead to an increase in the QRS-area, which is more pronounced in lead V3, compared to V6. These dynamics of the QRS-pattern are accompanied by the greatest rise in the ST-segment in lead V3. Although these changes in the QRS-area might be interpreted as a transition from one body position to another, the gradual time course of changes in the amplitude and slope of the ST-segment strongly suggest that these dynamics are caused by myocardial ischemia.
Presence of noise has also been proposed as a marker of changes in body position. Indeed, increased noise levels can be observed during the transition from one position to another in Figure 2 (upper rows). However, noise and changes in body position can be also associated with a patient's discomfort or chest pain during the ischemic events, and the high-frequency noise can be related to muscular activity and other factors. Moreover, patients with asymptomatic ischemic events might carry out different types of physical activity during the events. Thus, caution is required in the interpretation of noise as an indicator of positional changes.
In Figure 2, the development of the ST-segment elevation during the ischemic event spans approximately 5 min until reaching its maximum value. This time course of changes in the repolarization segment, although more gradual than that during the positional tests, was nevertheless relatively short compared to other patients in the studied group. In most patients, the microvolt-level, sub-threshold deviation of the ST-segment developed over 15-20 min, until it reached the maximum, super-threshold level. It is interesting to note that the patient whose data is shown in Figure 1 did not have significant obstructive coronary artery disease according to the results of cardiac catheterization, and the etiology of the ischemic event had been attributed to a possible coronary vasospasm, which could have explained the relatively short time course of the ischemic episode. A typical time course of development of an ischemic event in a 74-year old female is shown in Figure 3. The time series of the ST-amplitude in consecutive cardiac complexes during the ischemic event consists of approximately 15 min of sub-threshold (< 100 μV) ST-depression immediately followed by 5 min of super-threshold (>100 μV) ST-depression. The ischemic changes in repolarization segment were also accompanied by gradual dynamics of the QRS-area in leads V3 and V6 (Figure 3, Rows 5,6), and similar decrease in the T-wave amplitude and QT-interval (data not shown).
Changes in heart rate during ischemic events
Overall in the studied group, the cardiac cycle lengths gradually decreased 19% in the 15-20 min before the time point when the maximum deviation of the ST-segment amplitude is reached (p=0.033). These dynamics of cardiac rhythm during the event suggests that an increase of sympathetic activity accompanied ischemia development, possibly due to chest pain or patient's discomfort.
Continuous changes in the ST-segment on the RMS-curve
Due to the differences in ischemia localization, the overall changes in the ST-amplitude in the studied group in each individual lead were not statistically significant. However, despite these differences, the RMS-curve showed a gradual, significant increase in the amplitude of the ST-segment in the studied group (211%, p<0.017).
Conclusions
The main findings of this study were 1) the slow, gradual time course of development of ischemic changes in the repolarization segment (15-20 min) with an initial period of sub-threshold ischemia (<100 μV) followed by the development of super-threshold changes and 2) a slow increase in heart rate during these periods suggesting a gradual rise in sympathetic activity. The slow, gradual time course of development of these ECG changes creates a window of opportunity for early diagnosis and timely initiation of appropriate therapy. We have also observed that the ischemic changes in the amplitude of the ST-segment in the studied group were identifiable using the RMS-curve, although these dynamics were not statistically significant in the individual leads due to the differences in the ischemia localization. Finally, we have observed that the magnitude and area of the QRS-complex also exhibit gradual changes that accompany dynamics of the ST-segment (Figures 2, 3).
The changes in the QRS amplitude could have been caused by the development of myocardial ischemia, changes in body position, or a combination of these factors. For example, an ischemia-caused chest pain could have led to the changes in body position, which in turn, could generate the high-frequency muscle and movement artifacts. Therefore, reliable identification and dynamic tracking of the “true” ischemic ECG changes remains technically difficult and requires application of adaptive, multiparametric processing approaches. The time course of ECG changes during ischemia is more gradual than that during changes in body position. Therefore, the slow, simultaneous progression of changes in the amplitude of ST-segment and QRS-complex in Figures 2 and 3 strongly suggests that the changes in both depolarization and repolarization segments are due to the development of myocardial ischemia.
In this preliminary study, the magnitude of changes in the amplitude of the ST-segment during noise-free periods of stable body position was limited to less than 100 μV. This is not in disagreement with previous studies that also reported the average deviation of the ST-segment in different positions of < 100 μV.9 Hence, it is likely that changes in body position represent only one out of many physiological variables that affect the accuracy of ischemia monitoring. This provides further support for the notion that adaptive, multiparametric methods are necessary to improve the performance of ischemia detectors and reliably filter out false alarms.
We have extended previous adaptive methods by developing a neural network with a modified Hidden-Markov-model, which incorporates a broad range of parameters (primary elements) that completely describe the patterns and dynamics of the ECG waveforms.11,12,13 This method has been selected considering the advantages of the neural network in the learning and adapting to large, multiparametric datasets, and the hidden Markov models in determining the transitions between different states in multiparametric, noise-contaminated systems. Although the first preliminary results obtained in this small training set are promising, larger studies in heterogeneous patient populations are needed to improve and refine the neural network structure and the transition and emission probability matrices used in the hidden Markov model, and examine the accuracy of the ischemia detector in different patient populations.
Acknowledgments
Support: NHLBI R01 HL69753 (Dr. Drew), NHLBI, 1R43HL077116 (Dr. Shusterman).
Financial Associations: Dr. Shusterman has a significant (>5%) ownership interest in PinMed, Inc., Pittsburgh, PA. PinMed, Inc. has provided the software used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crawford M, Bernstein S, Deedwania P, et al. ACC/AHA Guidelines for ambulatory electrocardiography. J Am Coll Cardiol. 1999;34:912. doi: 10.1016/s0735-1097(99)00354-x. [DOI] [PubMed] [Google Scholar]
- 2.Norgaard BL, Rasmussen BM, Dellborg M, et al. Temporal and positional variability of the ST segment during continuous vectorcardiography monitoring in healthy subjects. J Electrocardiol. 1999;32:149. doi: 10.1016/s0022-0736(99)90093-6. [DOI] [PubMed] [Google Scholar]
- 3.Adams MG, Drew BJ. Efficacy of 2 Strategies to Detect Body Position ST-Segment Changes during Continuous 12-Lead Electrocardiographic Monitoring. Journal of Electrocardiology. 2002;35(Suppl):193. doi: 10.1054/jelc.2002.37181. [DOI] [PubMed] [Google Scholar]
- 4.Drew BJ, Adams MG. Clinical consequences of ST-segment changes caused by body position mimicking transient myocardial ischemia: hazards of ST-segment monitoring? J Electrocardiol. 2001 Jul;34(3):261. doi: 10.1054/jelc.2001.25431. [DOI] [PubMed] [Google Scholar]
- 5.García J, Åström M, Mendive J, Laguna P, Sörnmo L. ECG-Based Detection of Body Position Changes in Ischemia Monitoring IEEE Transactions on Biomedical Engineering. 2003;50:677. doi: 10.1109/TBME.2003.812208. [DOI] [PubMed] [Google Scholar]
- 6.Shusterman V, Shah SI, Beigel A, Anderson KP. Enhancing the precision of ECG baseline correction: selective filtering and removal of residual error. Computers and Biomedical Research. 2000;33:144–160. doi: 10.1006/cbmr.2000.1539. [DOI] [PubMed] [Google Scholar]
- 7.Shusterman V, Goldberg A. Tracking Repolarization Dynamics in Real-Life Data. J of Electrocardiology. 2004;37:180–186. doi: 10.1016/j.jelectrocard.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 8.Astrom M, Garcia J, Laguna P, Pahlm O, Sornmo L. Detection of body position changes using the surface electrocardiogram. Med. Biol. Eng. Comput. 2003;41:164. doi: 10.1007/BF02344884. [DOI] [PubMed] [Google Scholar]
- 9.García J, Sörnmo L, Olmos S, Laguna P. Automatic Detection of ST-T Complex Changes on the ECG Using Filtered RMS Difference Series: Application to Ambulatory Ischemia Monitoring, IEEE Transactions on Biomedical Engineering. 2000;47:1195. doi: 10.1109/10.867943. [DOI] [PubMed] [Google Scholar]
- 10.Jager F, Moody GB, Mark RG. Detection of Transient ST Segment Episodes During Ambulatory ECG Monitoring, Computers and Biomedical Research. 1998;31:305. doi: 10.1006/cbmr.1998.1483. [DOI] [PubMed] [Google Scholar]
- 11.Shusterman V. System and device for multi-scale analysis and representation of electrocardiographic data. U.S. Patent No 6,389,308 2000
- 12.Shusterman V. System and device for multi-scale analysis and representation of physiological data. U.S. Patent No 6,925,324 2002
- 13.Shusterman V, Trofimov O. Building and application of expert systems for differential diagnostics of cardiovascular diseases. Systems Analysis Modeling Simulation. 1994;14:15–24. [Google Scholar]
- 14.Shusterman V, Beigel A, Shah SI, Aysin B, Weiss R, Gottipaty VK, Schwartzman D, Anderson KP. Changes in autonomic activity and ventricular repolarization. Journal of Electrocardiology. 1999;32:185–192. doi: 10.1016/s0022-0736(99)90078-x. [DOI] [PubMed] [Google Scholar]