Abstract
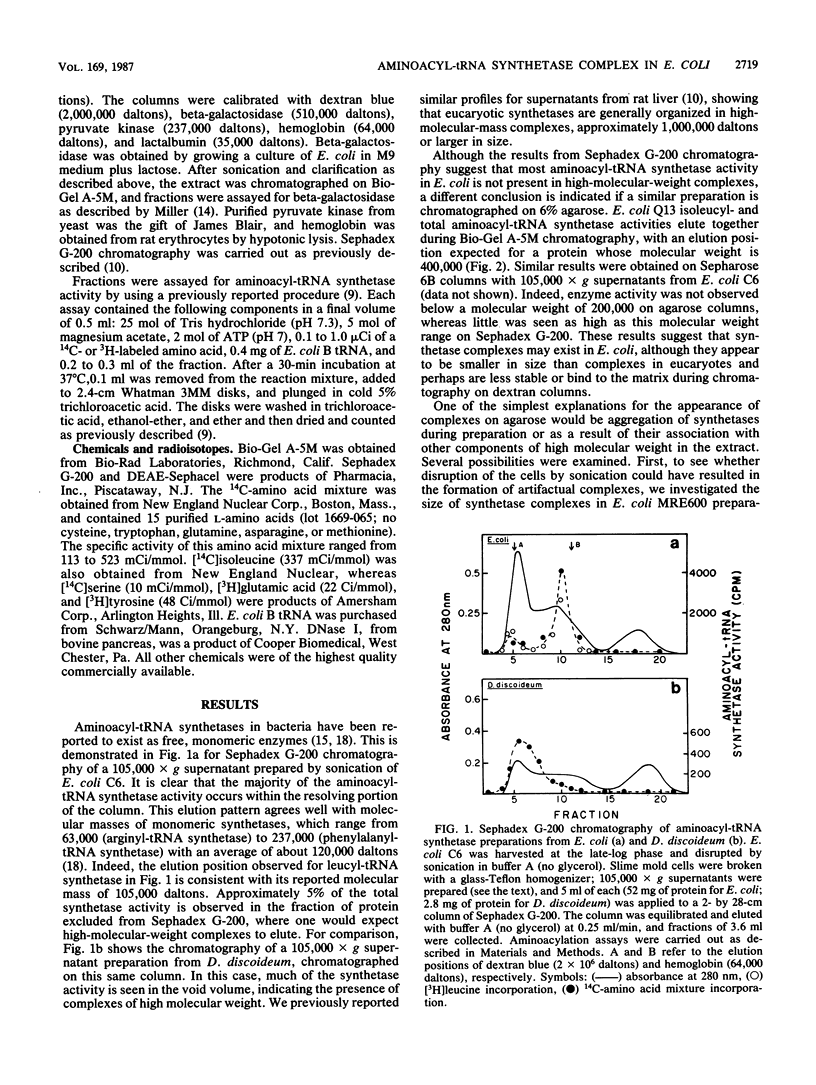
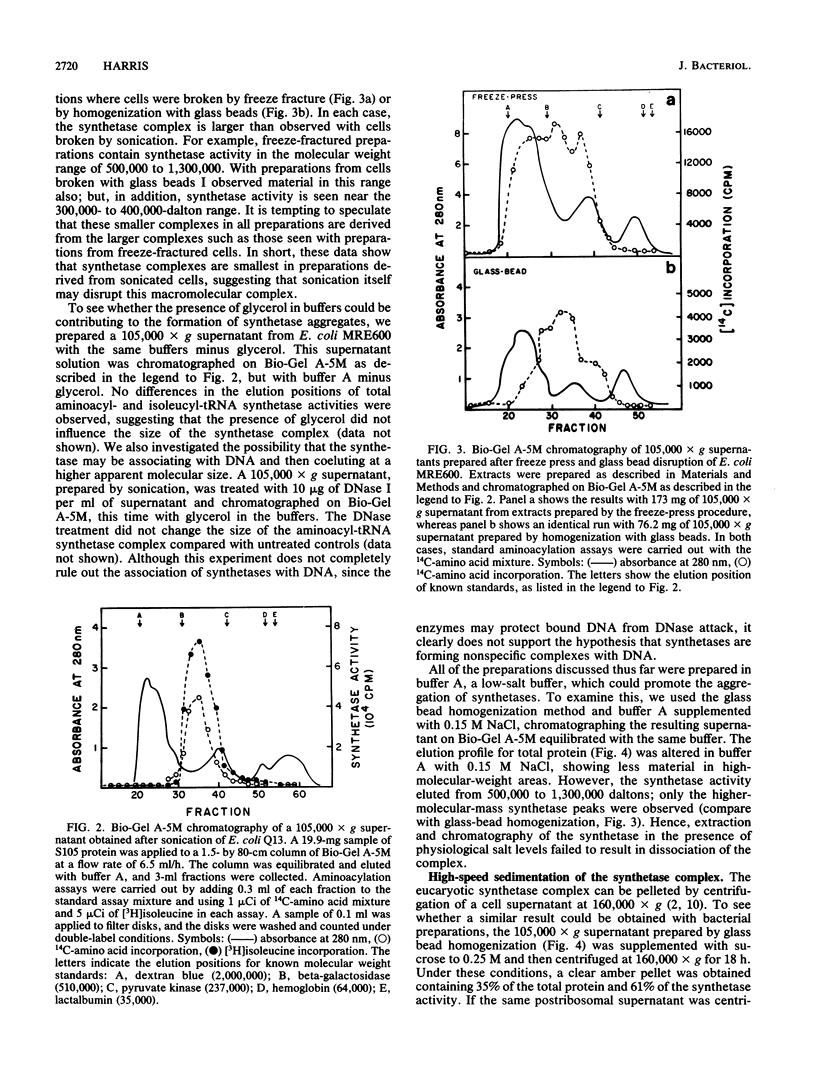
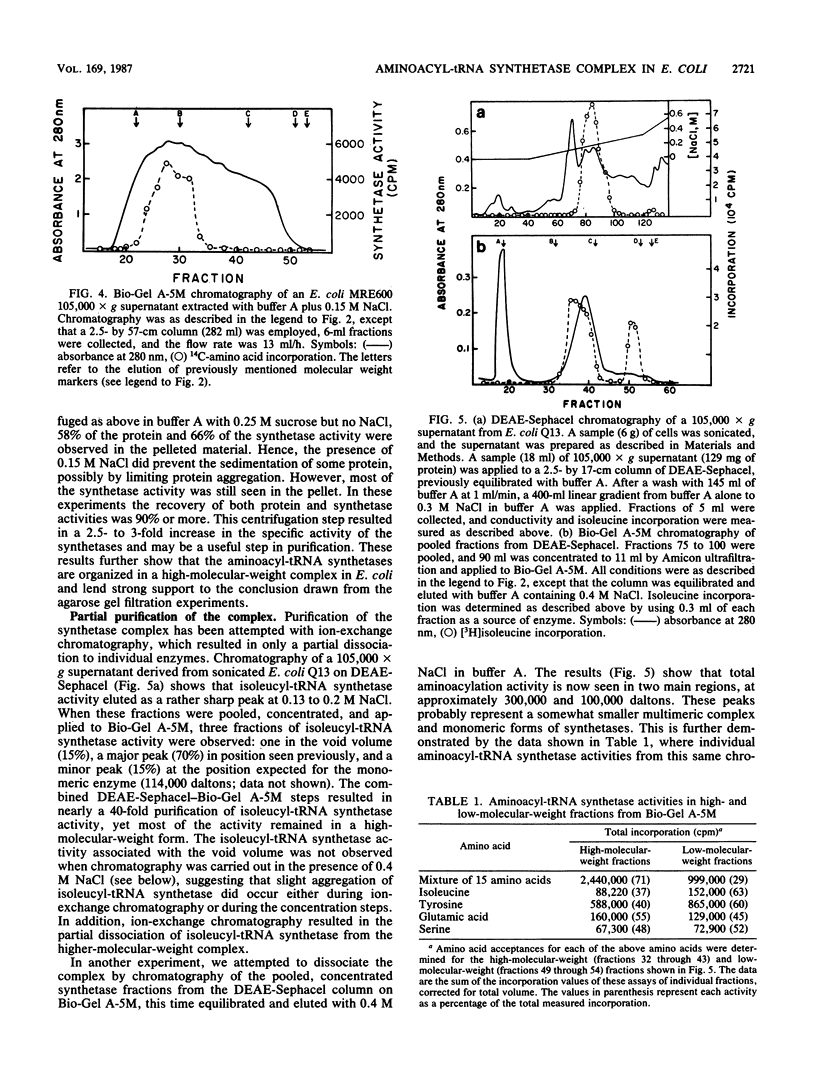
Aminoacyl-tRNA synthetases from several strains of Escherichia coli are shown to elute as a high-molecular-weight complex on 6% agarose columns (Bio-Gel A-5M). In contrast, very little synthetase activity was observed in such complexes on Sephadex G-200 columns, suggesting that these enzymes may interact with or are dissociated during chromatography on dextran. The size of the complex observed on Bio-Gel A-5M was influenced by the method of cell breakage and the salt concentrations present in buffers. The largest complexes (greater than 1,000,000 daltons) were seen with cells broken with a freeze press, whereas with sonicated preparations the average size of the complex was about 400,000 daltons. Extraction of synthetases at 0.15 M NaCl, to mimic physiological salt concentrations, also resulted in high-molecular-weight complexes, as demonstrated by both agarose gel filtration and ultracentrifugation analysis. Evidence is presented that dissociation of some synthetases does occur in the presence of higher salt levels (0.4 M NaCl). Partial purification of the synthetase complex on DEAE-Sephacel was accomplished with only minor dissociation of individual synthetases. These data suggest that a complex(es) of aminoacyl-tRNA synthetase does exist in bacterial cells, just as in eucaryotes, and that the complex may have escaped earlier detection due to its fragility during isolation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A. K., Deutscher M. P. Complex of aminoacyl-transfer RNA synthetases. J Mol Biol. 1971 Aug 28;60(1):113–122. doi: 10.1016/0022-2836(71)90451-7. [DOI] [PubMed] [Google Scholar]
- Brevet A., Kellermann O., Tonetti H., Waller J. P. Macromolecular complexes of aminoacyl-tRNA synthetases from eukaryotes. 2. Agarose gel-filtration behaviour of the extensively purified high-molecular-weight complex(es) of seven aminoacyl-tRNA synthetases from sheep liver. Eur J Biochem. 1979 Sep;99(3):551–558. doi: 10.1111/j.1432-1033.1979.tb13287.x. [DOI] [PubMed] [Google Scholar]
- Cirakoglu B., Waller J. P. Do yeast aminoacyl-tRNA synthetases exist as soluble enzymes within the cytoplasm? Eur J Biochem. 1985 Jun 3;149(2):353–361. doi: 10.1111/j.1432-1033.1985.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Johnson D. L., Yang D. C. High molecular mass amino acyl-tRNA synthetase complexes in eukaryotes. FEBS Lett. 1982 Jun 1;142(1):1–6. doi: 10.1016/0014-5793(82)80206-8. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P. The eucaryotic aminoacyl-tRNA synthetase complex: suggestions for its structure and function. J Cell Biol. 1984 Aug;99(2):373–377. doi: 10.1083/jcb.99.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EATON N. R. New press for disruption of microorganisms. J Bacteriol. 1962 Jun;83:1359–1360. doi: 10.1128/jb.83.6.1359-1360.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel A., Enger M. D. Subcellular distribution of aminoacyl-transfer RNA synthetases in Chinese hamster ovary cell culture. J Mol Biol. 1973 Sep 15;79(2):285–293. doi: 10.1016/0022-2836(73)90006-5. [DOI] [PubMed] [Google Scholar]
- Harris C. L., Lui L., Sakallah S., DeVore R. Cysteine starvation, isoleucyl-tRNAIle, and the regulation of the ilvGEDA operon of Escherichia coli. J Biol Chem. 1983 Jun 25;258(12):7676–7683. [PubMed] [Google Scholar]
- Harris C. L., Marin K., Stewart D. tRNA sulfurtransferase: a member of the aminoacyl-tRNA synthetase complex in rat liver. Biochem Biophys Res Commun. 1977 Dec 7;79(3):657–662. doi: 10.1016/0006-291x(77)91162-7. [DOI] [PubMed] [Google Scholar]
- Harris C. L., Titchener E. B., Cline A. L. Sulfur-deficient transfer ribonucleic acid in a cysteine-requiring, "relaxed" mutant of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1322–1327. doi: 10.1128/jb.100.3.1322-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann O., Brevet A., Tonetti H., Waller J. P. Macromolecular complexes of aminoacyl-tRNA synthetases from eukaryotes. 1. Extensive purification and characterization of the high-molecular-weight complex(es) of seven aminoacyl-tRNA synthetases from sheep liver. Eur J Biochem. 1979 Sep;99(3):541–550. doi: 10.1111/j.1432-1033.1979.tb13286.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nass G., Stöffler G. Molecular weight distribution of the aminoacyl-tRNA-synthetases of Escherichia coli by gel filtration. Mol Gen Genet. 1967;100(4):378–382. doi: 10.1007/BF00334065. [DOI] [PubMed] [Google Scholar]
- Pendergast A. M., Traugh J. A. Alteration of aminoacyl-tRNA synthetase activities by phosphorylation with casein kinase I. J Biol Chem. 1985 Sep 25;260(21):11769–11774. [PubMed] [Google Scholar]
- Saxholm H. J., Pitot H. C. Characterization of a proteolipid complex of aminoacyl-tRNA synthetases and transfer RNA from rat liver. Biochim Biophys Acta. 1979 May 24;562(3):386–399. doi: 10.1016/0005-2787(79)90103-5. [DOI] [PubMed] [Google Scholar]
- Schimmel P. R., Söll D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- Vennegoor C., Bloemendal H. Occurrence and particle character of aminoacyl-tRNA synthetases in the post-microsomal fraction from rat liver. Eur J Biochem. 1972 Apr 24;26(4):462–473. doi: 10.1111/j.1432-1033.1972.tb01788.x. [DOI] [PubMed] [Google Scholar]



