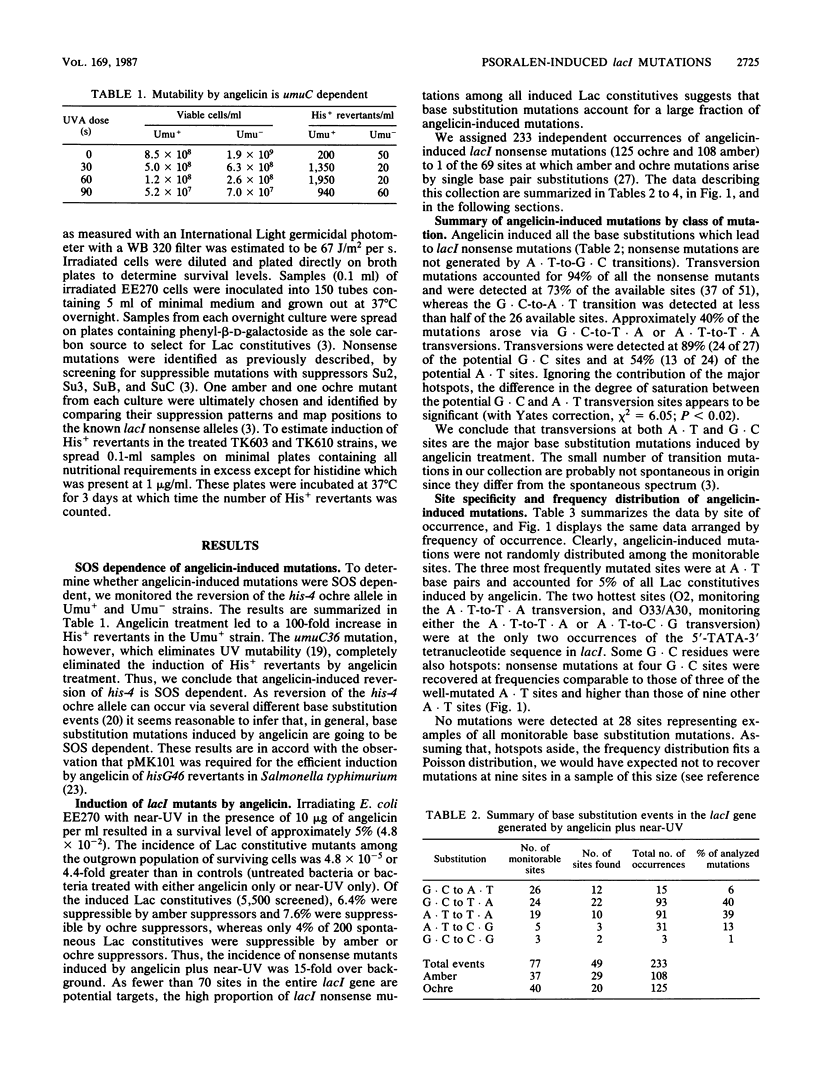
Abstract
Angelicin- plus near-UV-induced mutations were umuC dependent in Escherichia coli K-12. Angelicin, a monofunctional psoralen derivative, is believed to damage DNA almost exclusively at pyrimidine bases. To broaden our knowledge about the mutagenic specificity of SOS-dependent mutagens, we determined the mutational specificity of 233 suppressible lacI mutations induced by angelicin. More than 90% of the nonsense mutations arose via transversion substitutions. The three most frequently mutated sites were at A-T base pairs and accounted for more than one-third of all induced nonsense mutations. The two hottest sites were at the only occurrences of the 5'-TATA-3' tetranucleotide in lacI, a sequence expected to be a preferred binding site for a psoralen. Both A-T-to-T-A and A-T-to-C-G transversions were well induced by angelicin treatment, but the frequency of each transversion depended on the particular site. We also detected significant induction of transversion mutations at G-C sites. The induction of transversions by an SOS-dependent mutagen that generates lesions at pyrimidines supports the idea that DNA lesions influence the selection of bases that are incorporated via the process of SOS repair.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backendorf C., Spaink H., Barbeiro A. P., van de Putte P. Structure of the uvrB gene of Escherichia coli. Homology with other DNA repair enzymes and characterization of the uvrB5 mutation. Nucleic Acids Res. 1986 Apr 11;14(7):2877–2890. doi: 10.1093/nar/14.7.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F. Furocoumarin photochemistry and its main biological implications. Curr Probl Dermatol. 1986;15:137–163. [PubMed] [Google Scholar]
- Dall'Acqua F., Terbojevich M., Marciani S., Vedaldi D., Recher M. Investigation of the dark interaction between furocoumarins and DNA. Chem Biol Interact. 1978 Apr;21(1):103–115. doi: 10.1016/0009-2797(78)90071-6. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Vedaldi D., Caffieri S., Guiotto A., Bordin F., Rodighiero G. Chemical basis of the photosensitizing activity of angelicins. Natl Cancer Inst Monogr. 1984 Dec;66:55–60. [PubMed] [Google Scholar]
- Drake J. W., McGuire J. Properties of r mutants of bacteriophage T4 photodynamically induced in the presence of thiopyronin and psoralen. J Virol. 1967 Apr;1(2):260–267. doi: 10.1128/jvi.1.2.260-267.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt E., Warren A. J., Porter J., Atkins D., Miller J. H. Carcinogenic epoxides of benzo[a]pyrene and cyclopenta[cd]pyrene induce base substitutions via specific transversions. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1945–1949. doi: 10.1073/pnas.79.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt E., Wolf M., Goldberg I. H. Mutagenesis by neocarzinostatin in Escherichia coli and Salmonella typhimurium: requirement for umuC+ or plasmid pKM101. J Bacteriol. 1980 Nov;144(2):656–660. doi: 10.1128/jb.144.2.656-660.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Eisenstadt E., Cairns J. Random components in mutagenesis. Nature. 1982 Sep 23;299(5881):365–367. doi: 10.1038/299365a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Eisenstadt E. Distribution and specificity of mutations induced by neocarzinostatin in the lacI gene of Escherichia coli. J Bacteriol. 1983 Jan;153(1):379–383. doi: 10.1128/jb.153.1.379-383.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Eisenstadt E. Induction of transversion mutations in Escherichia coli by N-methyl-N'-nitro-N-nitrosoguanidine is SOS dependent. J Bacteriol. 1985 Jul;163(1):213–220. doi: 10.1128/jb.163.1.213-220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster P. L., Eisenstadt E., Miller J. H. Base substitution mutations induced by metabolically activated aflatoxin B1. Proc Natl Acad Sci U S A. 1983 May;80(9):2695–2698. doi: 10.1073/pnas.80.9.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. F., Hopkins R. L., Lasken R., Mhaskar D. N. The biochemical basis of 5-bromouracil- and 2-aminopurine-induced mutagenesis. Basic Life Sci. 1985;31:409–423. doi: 10.1007/978-1-4613-2449-2_25. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Kato T., Shinoura Y., Templin A., Clark A. J. Analysis of ultraviolet light-induced suppressor mutations in the strain of Escherichia coli K-12 AB1157: an implication for molecular mechanisms of UV mutagenesis. Mol Gen Genet. 1980;180(2):283–291. doi: 10.1007/BF00425840. [DOI] [PubMed] [Google Scholar]
- Kitagawa Y., Akaboshi E., Shinagawa H., Horii T., Ogawa H., Kato T. Structural analysis of the umu operon required for inducible mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4336–4340. doi: 10.1073/pnas.82.13.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Mutational specificity of depurination. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1494–1498. doi: 10.1073/pnas.81.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. E., Ames B. N. Classifying mutagens as to their specificity in causing the six possible transitions and transversions: a simple analysis using the Salmonella mutagenicity assay. Environ Mutagen. 1986;8(1):9–28. doi: 10.1002/em.2860080103. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Apurinic sites as mutagenic intermediates. Cell. 1985 Mar;40(3):483–484. doi: 10.1016/0092-8674(85)90191-6. [DOI] [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H. Carcinogens induce targeted mutations in Escherichia coli. Cell. 1982 Nov;31(1):5–7. doi: 10.1016/0092-8674(82)90398-1. [DOI] [PubMed] [Google Scholar]
- Perry K. L., Elledge S. J., Mitchell B. B., Marsh L., Walker G. C. umuDC and mucAB operons whose products are required for UV light- and chemical-induced mutagenesis: UmuD, MucA, and LexA proteins share homology. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4331–4335. doi: 10.1073/pnas.82.13.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J. G., Hearst J. E. Termination sites of the in vitro nick-translation reaction on DNA that had photoreacted with psoralen. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5540–5544. doi: 10.1073/pnas.80.18.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Decuyper-Debergh D., Gamper H. Mutagenesis of the lac promoter region in M13 mp10 phage DNA by 4'-hydroxymethyl-4,5',8-trimethylpsoralen. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7355–7359. doi: 10.1073/pnas.82.21.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Hearst J. Sites of termination of in vitro DNA synthesis on psoralen phototreated single-stranded templates. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 Sep;48(3):381–388. doi: 10.1080/09553008514551381. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Base substitution mutations induced in the cI gene of lambda phage by neocarzinostatin chromophore: correlation with depyrimidination hotspots at the sequence AGC. Nucleic Acids Res. 1986 Feb 11;14(3):1417–1426. doi: 10.1093/nar/14.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povirk L. F., Goldberg I. H. Endonuclease-resistant apyrimidinic sites formed by neocarzinostatin at cytosine residues in DNA: evidence for a possible role in mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3182–3186. doi: 10.1073/pnas.82.10.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran W. A., Cantor C. R. Mutagenic SOS repair of site-specific psoralen damage in plasmid pBR322. J Mol Biol. 1984 Sep 25;178(3):595–609. doi: 10.1016/0022-2836(84)90240-7. [DOI] [PubMed] [Google Scholar]
- Saffran W. A., Cantor C. R. The complete pattern of mutagenesis arising from the repair of site-specific psoralen crosslinks: analysis by oligonucleotide hybridization. Nucleic Acids Res. 1984 Dec 21;12(24):9237–9248. doi: 10.1093/nar/12.24.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Loeb L. A. Depurination causes mutations in SOS-induced cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1773–1777. doi: 10.1073/pnas.78.3.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell H. M., Sakore T. D., Jain S. C., Banerjee A., Bhandary K. K., Reddy B. S., Lozansky E. D. beta-kinked DNA--a structure that gives rise to drug intercalation and DNA breathing--and its wider significance in determining the premelting and melting behavior of DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):293–314. doi: 10.1101/sqb.1983.047.01.035. [DOI] [PubMed] [Google Scholar]
- Strauss B., Rabkin S., Sagher D., Moore P. The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie. 1982 Aug-Sep;64(8-9):829–838. doi: 10.1016/s0300-9084(82)80138-7. [DOI] [PubMed] [Google Scholar]
- Tessman J. W., Isaacs S. T., Hearst J. E. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. Conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985 Mar 26;24(7):1669–1676. doi: 10.1021/bi00328a015. [DOI] [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Complementary base pairing and the origin of substitution mutations. Nature. 1976 Sep 23;263(5575):285–289. doi: 10.1038/263285a0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatagai F., Glickman B. W. Mutagenesis by 8-methoxypsoralen plus near-UV treatment: analysis of specificity in the lacI gene of Escherichia coli. Mutat Res. 1986 Dec;163(3):209–224. doi: 10.1016/0027-5107(86)90019-9. [DOI] [PubMed] [Google Scholar]
- de Mol N. J., Beijersbergen van Henegouwen G. M., Mohn G. R., Glickman B. W., van Kleef P. M. On the involvement of singlet oxygen in mutation induction by 8-methoxypsoralen and UVA irradiation in Escherichia coli K-12. Mutat Res. 1981 Jun;82(1):23–30. doi: 10.1016/0027-5107(81)90134-2. [DOI] [PubMed] [Google Scholar]


