Abstract
The recA gene of Synechococcus sp. strain PCC 7002 was detected and cloned from a lambda gtwes genomic library by heterologous hybridization by using a gene-internal fragment of the Escherichia coli recA gene as the probe. The gene encodes a 38-kilodalton polypeptide which is antigenically related to the RecA protein of E. coli. The nucleotide sequence of a portion of the gene was determined. The translation of this region was 55% homologous to the E. coli protein; allowances for conservative amino acid replacements yield a homology value of about 74%. The cyanobacterial recA gene product was proficient in restoring homologous recombination and partial resistance to UV irradiation to recA mutants of E. coli. Heterologous hybridization experiments, in which the Synechococcus sp. strain PCC 7002 recA gene was used as the probe, indicate that a homologous gene is probably present in all cyanobacterial strains.
Full text
PDF

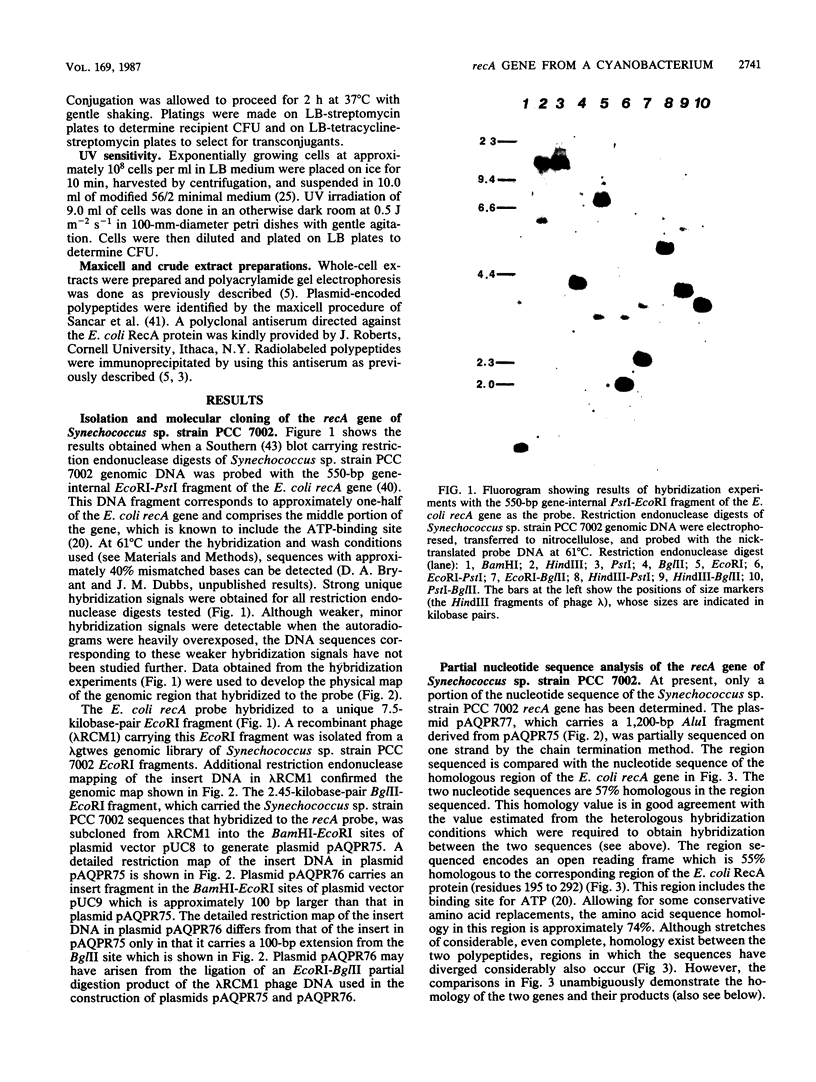






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Helinski D. R. Isolation and characterization of the recA gene of Rhizobium meliloti. J Bacteriol. 1983 Jul;155(1):311–316. doi: 10.1128/jb.155.1.311-316.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. A., de Lorimier R., Lambert D. H., Dubbs J. M., Stirewalt V. L., Stevens S. E., Jr, Porter R. D., Tam J., Jay E. Molecular cloning and nucleotide sequence of the alpha and beta subunits of allophycocyanin from the cyanelle genome of Cyanophora paradoxa. Proc Natl Acad Sci U S A. 1985 May;82(10):3242–3246. doi: 10.1073/pnas.82.10.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Expression of the Escherichia coli lacZ gene on a plasmid vector in a cyanobacterium. Science. 1985 Nov 15;230(4727):805–807. doi: 10.1126/science.2997920. [DOI] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Plasmid transformation in Agmenellum quadruplicatum PR-6: construction of biphasic plasmids and characterization of their transformation properties. J Bacteriol. 1983 Jun;154(3):1446–1450. doi: 10.1128/jb.154.3.1446-1450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Eitner G., Adler B., Lanzov V. A., Hofemeister J. Interspecies recA protein substitution in Escherichia coli and Proteus mirabilis. Mol Gen Genet. 1982;185(3):481–486. doi: 10.1007/BF00334144. [DOI] [PubMed] [Google Scholar]
- Finch P. W., Brough C. L., Emmerson P. T. Molecular cloning of a recA-like gene of Methylophilus methylotrophus and identification of its product. Gene. 1986;44(1):47–53. doi: 10.1016/0378-1119(86)90041-7. [DOI] [PubMed] [Google Scholar]
- Genthner F. J., Wall J. D. Isolation of a recombination-deficient mutant of Rhodopseudomonas capsulata. J Bacteriol. 1984 Dec;160(3):971–975. doi: 10.1128/jb.160.3.971-975.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan C. M., Houghton J. A. Molecular cloning and isolation of a cyanobacterial gene which increases the UV and methyl methanesulphonate survival of recA strains of Escherichia coli K12. J Gen Microbiol. 1987 Jan;133(1):119–126. doi: 10.1099/00221287-133-1-119. [DOI] [PubMed] [Google Scholar]
- Gilman S. C., Docherty J. J., Clarke A., Rawls W. E. Reaction patterns of herpes simplex virus type 1 and type 2 proteins with sera of patients with uterine cervical carcinoma and matched controls. Cancer Res. 1980 Dec;40(12):4640–4647. [PubMed] [Google Scholar]
- Ginsburg H., Edmiston S. H., Harper J., Mount D. W. Isolation and characterization of an operator-constitutive mutation in the recA gene of E. coli K-12. Mol Gen Genet. 1982;187(1):4–11. doi: 10.1007/BF00384376. [DOI] [PubMed] [Google Scholar]
- Goldberg I., Mekalanos J. J. Cloning of the Vibrio cholerae recA gene and construction of a Vibrio cholerae recA mutant. J Bacteriol. 1986 Mar;165(3):715–722. doi: 10.1128/jb.165.3.715-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Methods Enzymol. 1983;100:333–342. doi: 10.1016/0076-6879(83)00066-x. [DOI] [PubMed] [Google Scholar]
- Keener S. L., McNamee K. P., McEntee K. Cloning and characterization of recA genes froM Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli B/r. J Bacteriol. 1984 Oct;160(1):153–160. doi: 10.1128/jb.160.1.153-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K. L., McEntee K. Nucleotide binding by a 24-residue peptide from the RecA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9289–9293. doi: 10.1073/pnas.83.24.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokjohn T. A., Miller R. V. Molecular cloning and characterization of the recA gene of Pseudomonas aeruginosa PAO. J Bacteriol. 1985 Aug;163(2):568–572. doi: 10.1128/jb.163.2.568-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey J. M., Falkow S. Cloning of the recA gene of Neisseria gonorrhoeae and construction of gonococcal recA mutants. J Bacteriol. 1987 Feb;169(2):790–795. doi: 10.1128/jb.169.2.790-795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Lovett C. M., Jr, Roberts J. W. Purification of a RecA protein analogue from Bacillus subtilis. J Biol Chem. 1985 Mar 25;260(6):3305–3313. [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B., Porter D. D. Modes of gene transfer and recombination in bacteria. Annu Rev Genet. 1978;12:249–287. doi: 10.1146/annurev.ge.12.120178.001341. [DOI] [PubMed] [Google Scholar]
- Person S., Warner S. C., Bzik D. J., Debroy C., Fox B. A. Expression in bacteria of gB-glycoprotein-coding sequences of Herpes simplex virus type 2. Gene. 1985;35(3):279–287. doi: 10.1016/0378-1119(85)90006-x. [DOI] [PubMed] [Google Scholar]
- Porter R. D., Buzby J. S., Pilon A., Fields P. I., Dubbs J. M., Stevens S. E., Jr Genes from the cyanobacterium Agmenellum quadruplicatum isolated by complementation: characterization and production of merodiploids. Gene. 1986;41(2-3):249–260. doi: 10.1016/0378-1119(86)90105-8. [DOI] [PubMed] [Google Scholar]
- Porter R. D. Enhanced recombination between F42lac and lambda plac5: dependence on F42lac fertility functions. Mol Gen Genet. 1981;184(3):355–358. doi: 10.1007/BF00352504. [DOI] [PubMed] [Google Scholar]
- Porter R. D., McLaughlin T., Low B. Transduction versus "conjuduction": evidence for multiple roles for exonuclease V in genetic recombination in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1043–1047. doi: 10.1101/sqb.1979.043.01.113. [DOI] [PubMed] [Google Scholar]
- Porter R. D. Transformation in cyanobacteria. Crit Rev Microbiol. 1986;13(2):111–132. doi: 10.3109/10408418609108736. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Seifert H. S., Porter R. D. Enhanced recombination between lambda plac5 and mini-F-lac: the tra regulon is required for recombination enhancement. Mol Gen Genet. 1984;193(2):269–274. doi: 10.1007/BF00330679. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevens S. E., Porter R. D. Transformation in Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6052–6056. doi: 10.1073/pnas.77.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau de Marsac N., Borrias W. E., Kuhlemeier C. J., Castets A. M., van Arkel G. A., van den Hondel C. A. A new approach for molecular cloning in cyanobacteria: cloning of an Anacystis nidulans met gene using a Tn901-induced mutant. Gene. 1982 Nov;20(1):111–119. doi: 10.1016/0378-1119(82)90092-0. [DOI] [PubMed] [Google Scholar]
- Volkert M. R., Spencer D. F., Clark A. J. Indirect and intragenic suppression of the lexA102 mutation in E. coli B/r. Mol Gen Genet. 1979;177(1):129–137. doi: 10.1007/BF00267262. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Szalay A. A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1983 Sep;24(1):37–51. doi: 10.1016/0378-1119(83)90129-4. [DOI] [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y., Clewell D. B. Recombination-deficient mutant of Streptococcus faecalis. J Bacteriol. 1980 Aug;143(2):966–970. doi: 10.1128/jb.143.2.966-970.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey S. D., Porter R. D. General recombination in Escherichia coli K-12: in vivo role of RecBC enzyme. J Bacteriol. 1985 Apr;162(1):29–34. doi: 10.1128/jb.162.1.29-34.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorimier R., Bryant D. A., Porter R. D., Liu W. Y., Jay E., Stevens S. E., Jr Genes for the alpha and beta subunits of phycocyanin. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7946–7950. doi: 10.1073/pnas.81.24.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]