Abstract
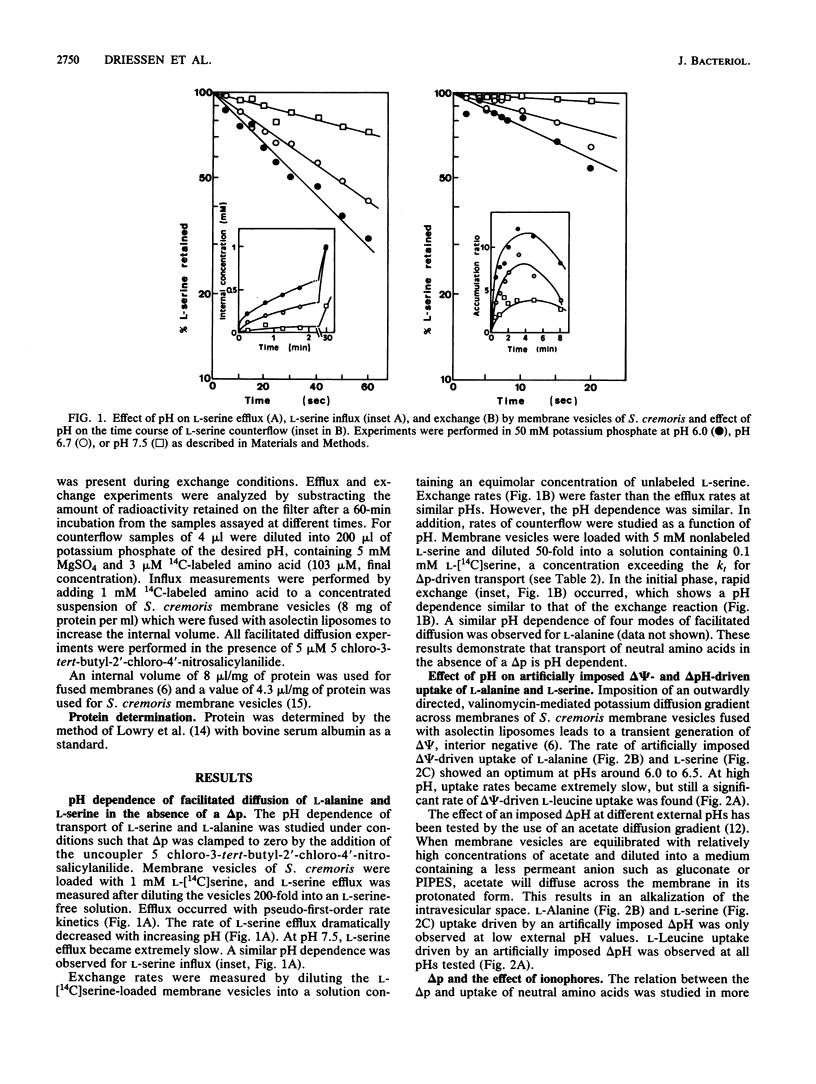
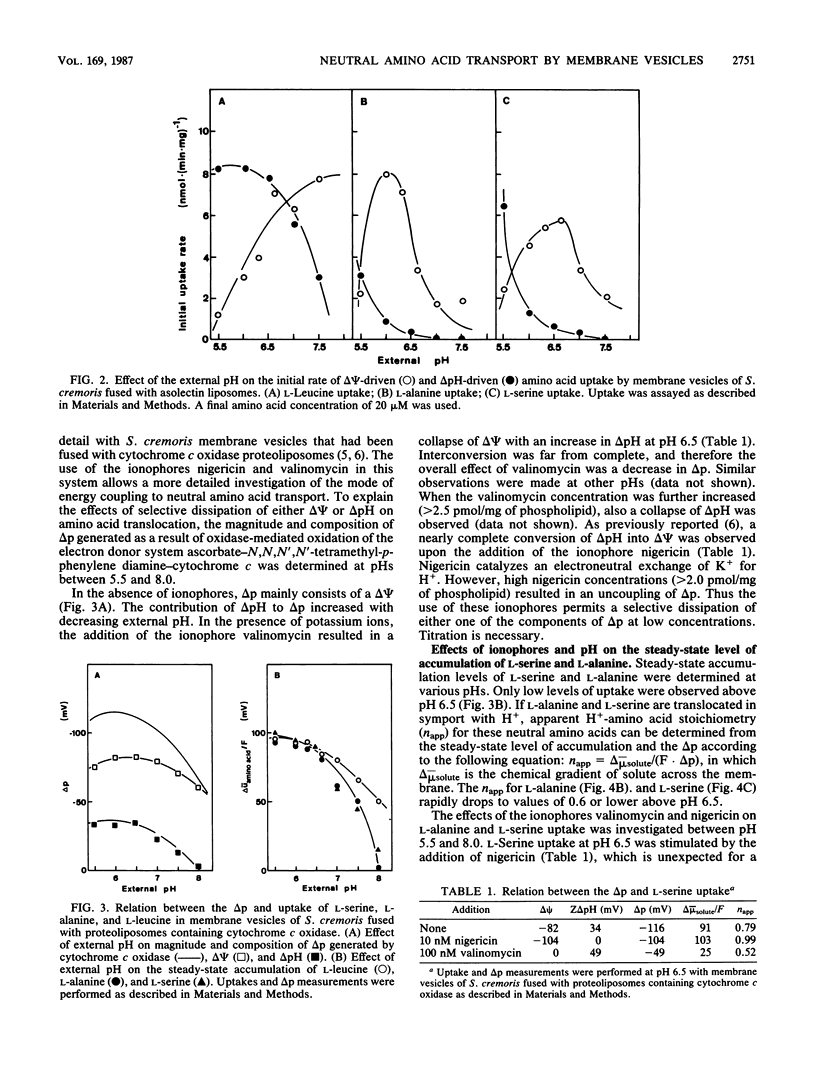
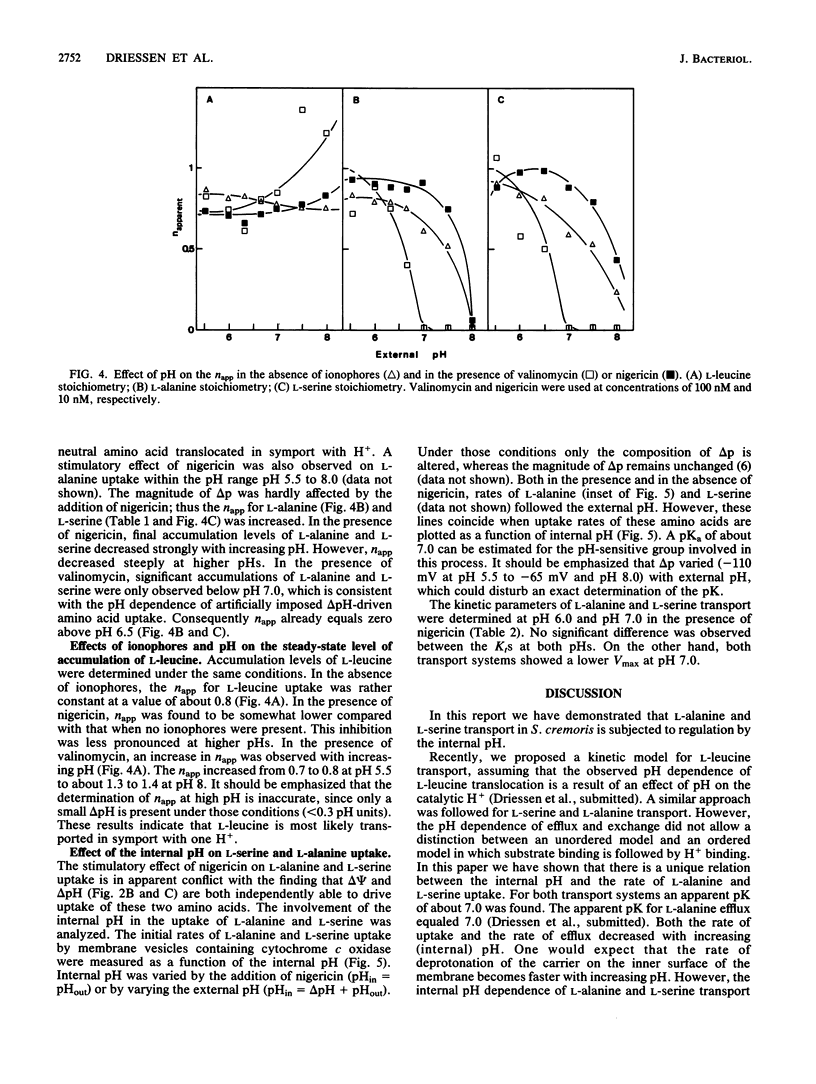
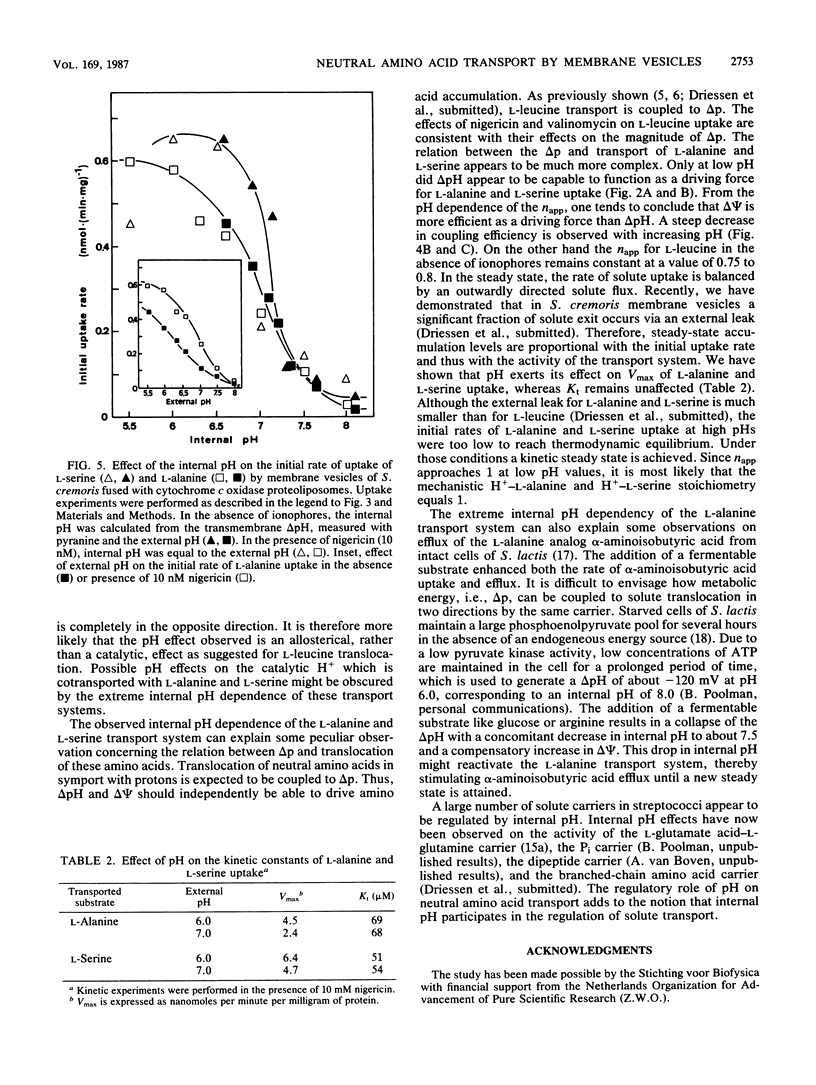
The pH dependence of transport of the neutral amino acids L-serine and L-alanine by membrane vesicles of Streptococcus cremoris have been studied in detail. The rates of four modes of facilitated diffusion (e.g., influx, efflux, exchange, and counterflow) of L-serine and L-alanine increase with increasing H+ concentration. Rates of artificially imposed electrical potential across the membrane (delta psi)-driven transport of L-serine and L-alanine show an optimum at pH 6 to 6.5. Under similar conditions, delta psi- and pH gradient across the membrane (delta pH)-driven transport of L-leucine is observed within the pH range studied (pH 5.5 to 7.5). The effect of ionophores on the uptake of L-alanine and L-serine has been studied in membrane vesicles of S. cremoris fused with proteoliposomes containing beef heart mitochondrial cytochrome c oxidase as a proton motive force (delta p)-generating system (Driessen et al., Proc. Natl. Acad. Sci. USA 82:7555-7559, 1985). An increase in the initial rates of L-serine and L-alanine uptake is observed with decreasing pH, which is not consistent with the pH dependency of delta p. Nigericin, an ionophore that induced a nearly complete interconversion of delta pH into delta psi, stimulated both the rate and the final level of L-alanine and L-serine uptake. Valinomycin, an ionophore that induced a collapse of delta psi with a noncompensating increase in delta pH, inhibited L-alanine and L-serine uptake above pH 6.0 more efficiently than it decreased delta p. Experiments which discriminate between the effects of the internal pH and the driving force (delta pH) on solute transport indicate that at high internal pH the transport systems for L-alanine and L-serine are inactivated. A unique relation exists between the internal pH and the initial rate of uptake of L-serine and L-alanine with an apparent pK of 7.0. The rate of L-alanine and L-serine uptake decreases with increasing internal pH. The apparent complex relation between the delta p and transport of L-alanine and L-serine can be explained by a regulatory effect of the internal pH on the activity of the L-serine and L-alanine carriers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., Mangerich W. E. The effects of weak acids on potassium uptake by Escherichia coli K-12 inhibition by low cytoplasmic pH. Biochim Biophys Acta. 1983 May 5;730(2):379–386. doi: 10.1016/0005-2736(83)90355-3. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Incorporation of beef heart cytochrome c oxidase as a proton-motive force-generating mechanism in bacterial membrane vesicles. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7555–7559. doi: 10.1073/pnas.82.22.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst P., Solioz M. The vanadate-sensitive ATPase of Streptococcus faecalis pumps potassium in a reconstituted system. J Biol Chem. 1986 Mar 25;261(9):4302–4308. [PubMed] [Google Scholar]
- Harold F. M., Kakinuma Y. Primary and secondary transport of cations in bacteria. Ann N Y Acad Sci. 1985;456:375–383. doi: 10.1111/j.1749-6632.1985.tb14888.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. J Biol Chem. 1985 Jan 10;260(1):72–76. [PubMed] [Google Scholar]
- Kobayashi H., Murakami N., Unemoto T. Regulation of the cytoplasmic pH in Streptococcus faecalis. J Biol Chem. 1982 Nov 25;257(22):13246–13252. [PubMed] [Google Scholar]
- Kobayashi H., Suzuki T., Unemoto T. Streptococcal cytoplasmic pH is regulated by changes in amount and activity of a proton-translocating ATPase. J Biol Chem. 1986 Jan 15;261(2):627–630. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Hellingwerf K. J., Konings W. N. Regulation of the glutamate-glutamine transport system by intracellular pH in Streptococcus lactis. J Bacteriol. 1987 May;169(5):2272–2276. doi: 10.1128/jb.169.5.2272-2276.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinbo T., Kamo N., Kurihara K., Kobatake Y. A PVC-based electrode sensitive to DDA+ as a device for monitoring the membrane potential in biological systems. Arch Biochem Biophys. 1978 Apr 30;187(2):414–422. doi: 10.1016/0003-9861(78)90052-8. [DOI] [PubMed] [Google Scholar]
- Thompson J. Characteristics and energy requirements of an alpha-aminoisobutyric acid transport system in Streptococcus lactis. J Bacteriol. 1976 Aug;127(2):719–730. doi: 10.1128/jb.127.2.719-730.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Yu L., King T. E. Studies on cytochrome oxidase. Interactions of the cytochrome oxidase protein with phospholipids and cytochrome c. J Biol Chem. 1975 Feb 25;250(4):1383–1392. [PubMed] [Google Scholar]
- de Vrij W., Driessen A. J., Hellingwerf K. J., Konings W. N. Measurements of the proton motive force generated by cytochrome c oxidase from Bacillus subtilis in proteoliposomes and membrane vesicles. Eur J Biochem. 1986 Apr 15;156(2):431–440. doi: 10.1111/j.1432-1033.1986.tb09600.x. [DOI] [PubMed] [Google Scholar]



