Abstract
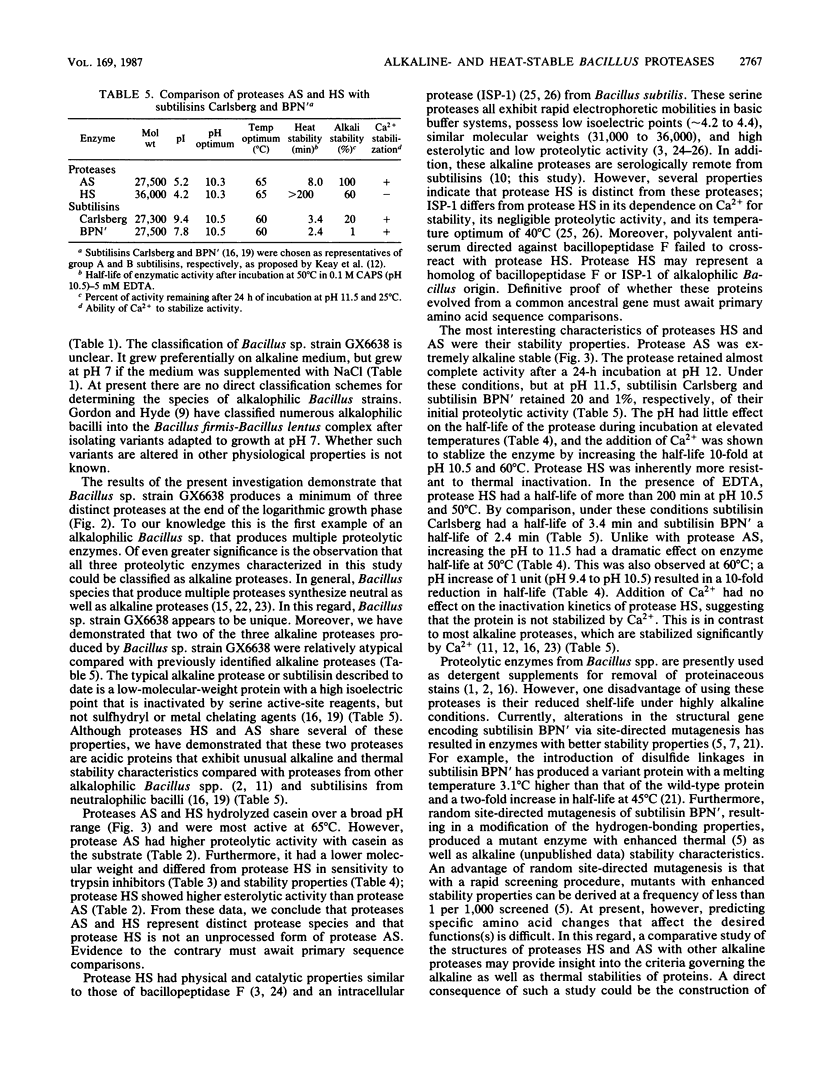
An alkalophilic Bacillus sp., strain GX6638 (ATCC 53278), was isolated from soil and shown to produce a minimum of three alkaline proteases. The proteases were purified by ion-exchange chromatography and were distinguishable by their isoelectric point, molecular weight, and electrophoretic mobility. Two of the proteases, AS and HS, which exhibited the greatest alkaline and thermal stability, were characterized further. Protease HS had an apparent molecular weight of 36,000 and an isoelectric point of approximately 4.2, whereas protease AS had a molecular weight of 27,500 and an isoelectric point of 5.2. Both enzymes had optimal proteolytic activities over a broad pH range (pH 8 to 12) and exhibited temperature optima of 65 degrees C. Proteases HS and AS were further distinguished by their proteolytic activities, esterolytic activities, sensitivity to inhibitors, and their alkaline and thermal stability properties. Protease AS was extremely alkali stable, retaining 88% of initial activity at pH 12 over a 24-h incubation period at 25 degrees C; protease HS exhibited similar alkaline stability properties to pH 11. In addition, protease HS had exceptional thermal stability properties. At pH 9.5 (0.1 M CAPS buffer, 5 mM EDTA), the enzyme had a half-life of more than 200 min at 50 degrees C and 25 min at 60 degrees C. At pH above 9.5, protease HS readily lost enzymatic activity even in the presence of exogenously supplied Ca2+. In contrast, protease AS was more stable at pH above 9.5, and Ca2+ addition extended the half-life of the enzyme 10-fold at 60 degrees C. In contrast, protease AS was more stable at pH above 9.5, and Ca2+ addition extended the half-life of the enzyme 10-fold at 60 degrees C. The data presented here clearly indicate that these two alkaline proteases from Bacillus sp. strain GX6638 represent novel proteases that differ fundamentally from the proteases previously described for members of the genus Bacillus.
Full text
PDF

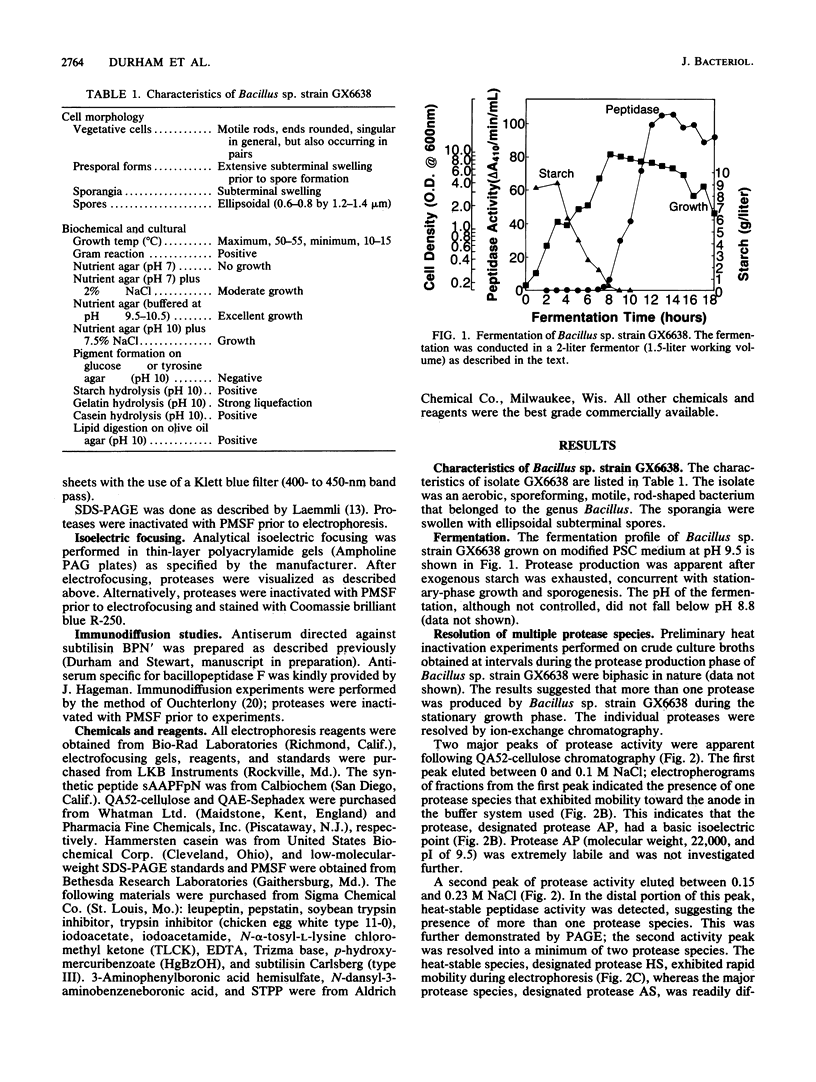
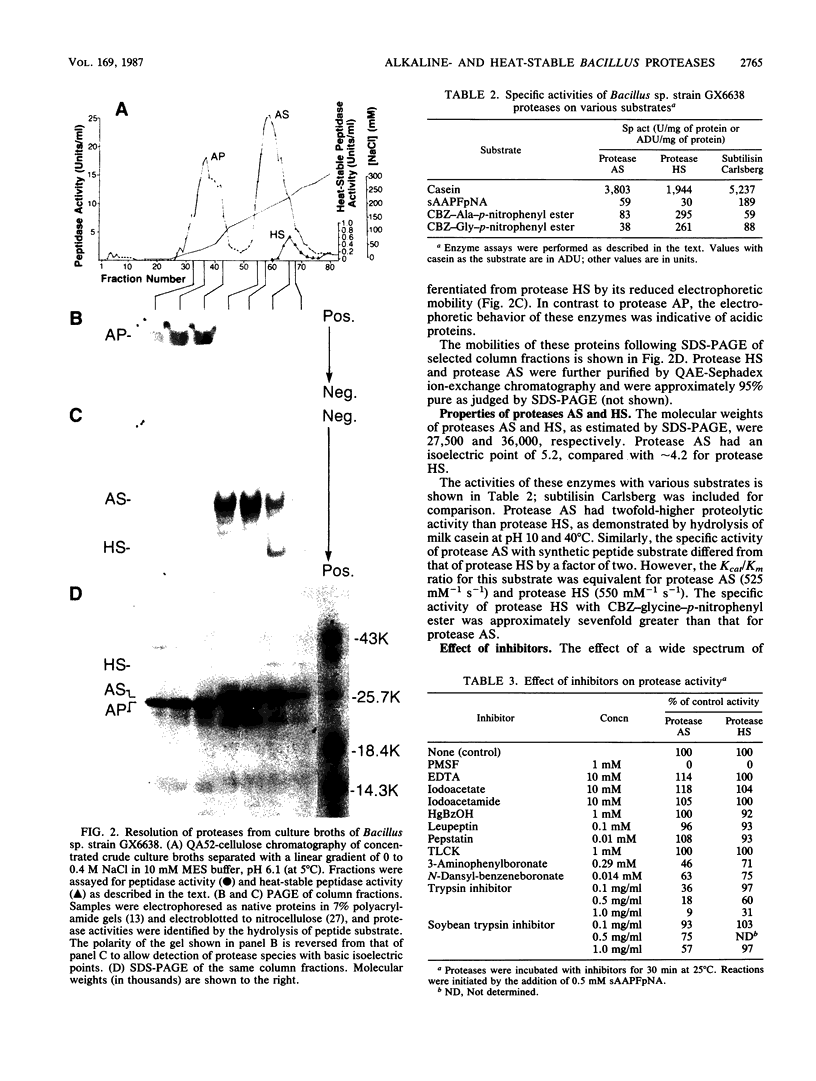
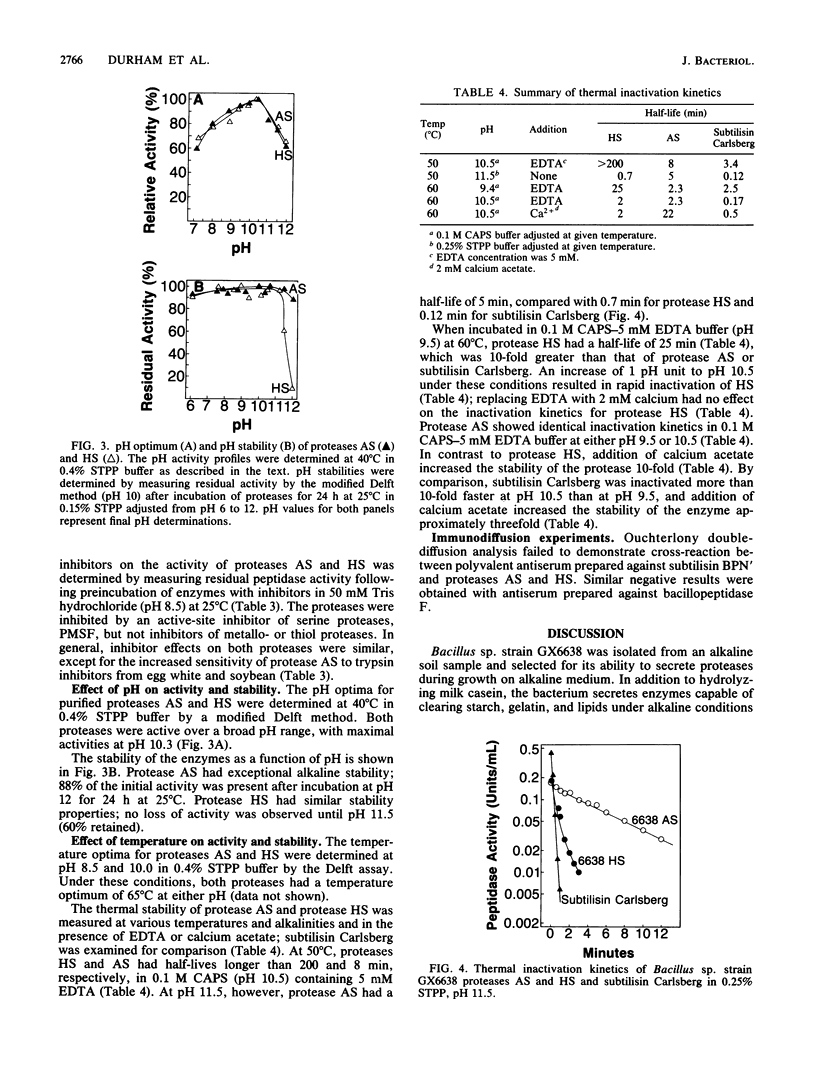

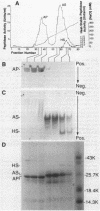
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Carlton B. C. Production of two proteolytic enzymes by a transformable strain of Bacillus subtilis. Arch Biochem Biophys. 1968 Nov;128(2):442–455. doi: 10.1016/0003-9861(68)90050-7. [DOI] [PubMed] [Google Scholar]
- Bryan P. N., Rollence M. L., Pantoliano M. W., Wood J., Finzel B. C., Gilliland G. L., Howard A. J., Poulos T. L. Proteases of enhanced stability: characterization of a thermostable variant of subtilisin. Proteins. 1986 Dec;1(4):326–334. doi: 10.1002/prot.340010406. [DOI] [PubMed] [Google Scholar]
- DelMar E. G., Largman C., Brodrick J. W., Geokas M. C. A sensitive new substrate for chymotrypsin. Anal Biochem. 1979 Nov 1;99(2):316–320. doi: 10.1016/s0003-2697(79)80013-5. [DOI] [PubMed] [Google Scholar]
- Estell D. A., Graycar T. P., Wells J. A. Engineering an enzyme by site-directed mutagenesis to be resistant to chemical oxidation. J Biol Chem. 1985 Jun 10;260(11):6518–6521. [PubMed] [Google Scholar]
- Hageman J. H., Carlton B. C. An enzymatic and immunological comparison of two proteases from a transformable Bacillus subtilis with the "subtilisins". Arch Biochem Biophys. 1970 Jul;139(1):67–79. doi: 10.1016/0003-9861(70)90045-7. [DOI] [PubMed] [Google Scholar]
- Keay L., Moser P. W., Wildi B. S. Proteases of the genus Bacillus. II. Alkaline proteases. Biotechnol Bioeng. 1970 Mar;12(2):213–249. doi: 10.1002/bit.260120206. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mäntsälä P., Zalkin H. Extracellular and membrane-bound proteases from Bacillus subtilis. J Bacteriol. 1980 Feb;141(2):493–501. doi: 10.1128/jb.141.2.493-501.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedkov P., Oberthür W., Braunitzer G. Determination of the complete amino-acid sequence of subtilisin DY and its comparison with the primary structures of the subtilisins BPN', Carlsberg and amylosacchariticus. Biol Chem Hoppe Seyler. 1985 Apr;366(4):421–430. doi: 10.1515/bchm3.1985.366.1.421. [DOI] [PubMed] [Google Scholar]
- Ng F. M., Dawes E. A. Chemostat studies on the regulation of glucose metabolism in Pseudomonas aeruginosa by citrate. Biochem J. 1973 Feb;132(2):129–140. doi: 10.1042/bj1320129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoliano M. W., Ladner R. C., Bryan P. N., Rollence M. L., Wood J. F., Poulos T. L. Protein engineering of subtilisin BPN': enhanced stabilization through the introduction of two cysteines to form a disulfide bond. Biochemistry. 1987 Apr 21;26(8):2077–2082. doi: 10.1021/bi00382a002. [DOI] [PubMed] [Google Scholar]
- Prestidge L., Gage V., Spizizen J. Protease activities during the course of sporulation on Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):815–823. doi: 10.1128/jb.107.3.815-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch C. A., Hageman J. H. Bacillopeptidase F: two forms of a glycoprotein serine protease from Bacillus subtilis 168. J Bacteriol. 1983 Jul;155(1):145–152. doi: 10.1128/jb.155.1.145-152.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin A. Y., Gorodetsky D. I., Kuznetsova I. A., Yanonis V. V., Abramov Z. T., Belyanova L. P., Baratova L. A., Stepanov V. M. Intracellular serine proteinase of Bacillus subtilis strain Marburg 168. Comparison with the homologous enzyme from Bacillus subtilis strain A-50. Biochem J. 1979 May 1;179(2):333–339. doi: 10.1042/bj1790333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin A. Y., Izotova L. S., Abramov Z. T., Ermakova L. M., Gorodetsky D. I., Stepanov V. M. On the appearance of Bacillus subtilis intracellular serine protease in the cell membrane and culture medium. Comparison of the enzyme and other Bacillus subtilis serine proteases. Arch Microbiol. 1978 Dec 20;119(3):287–293. doi: 10.1007/BF00405408. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]