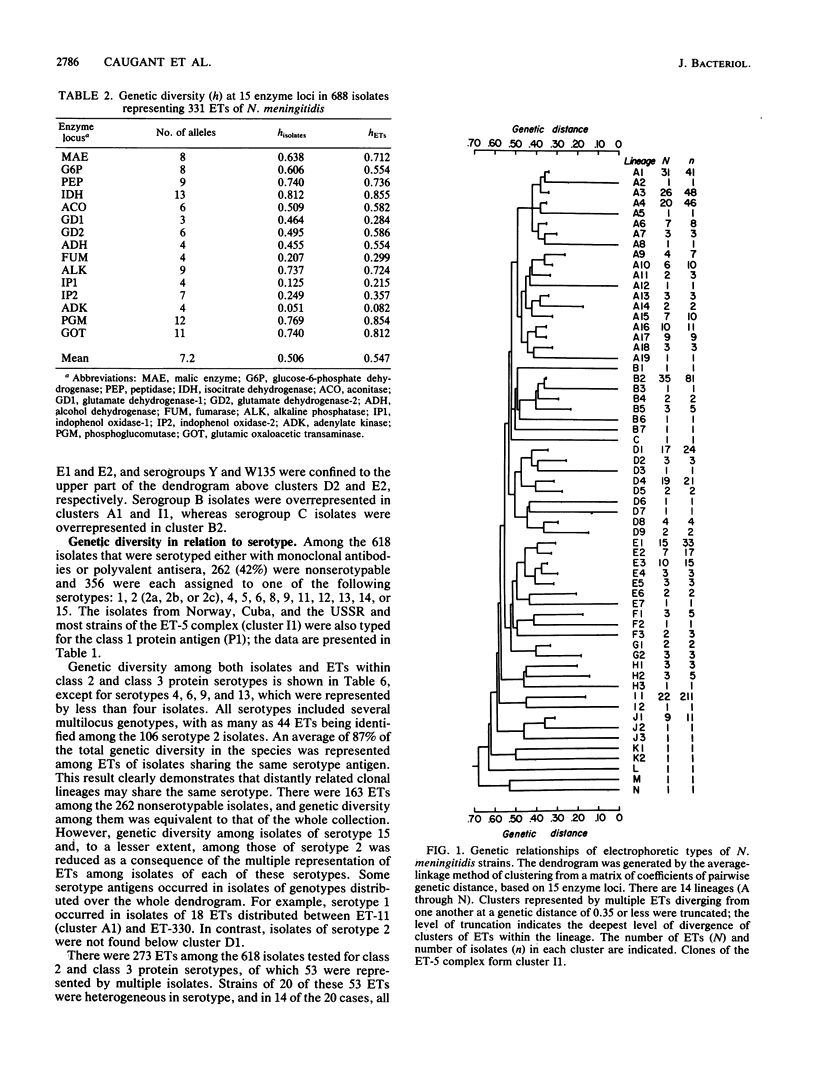
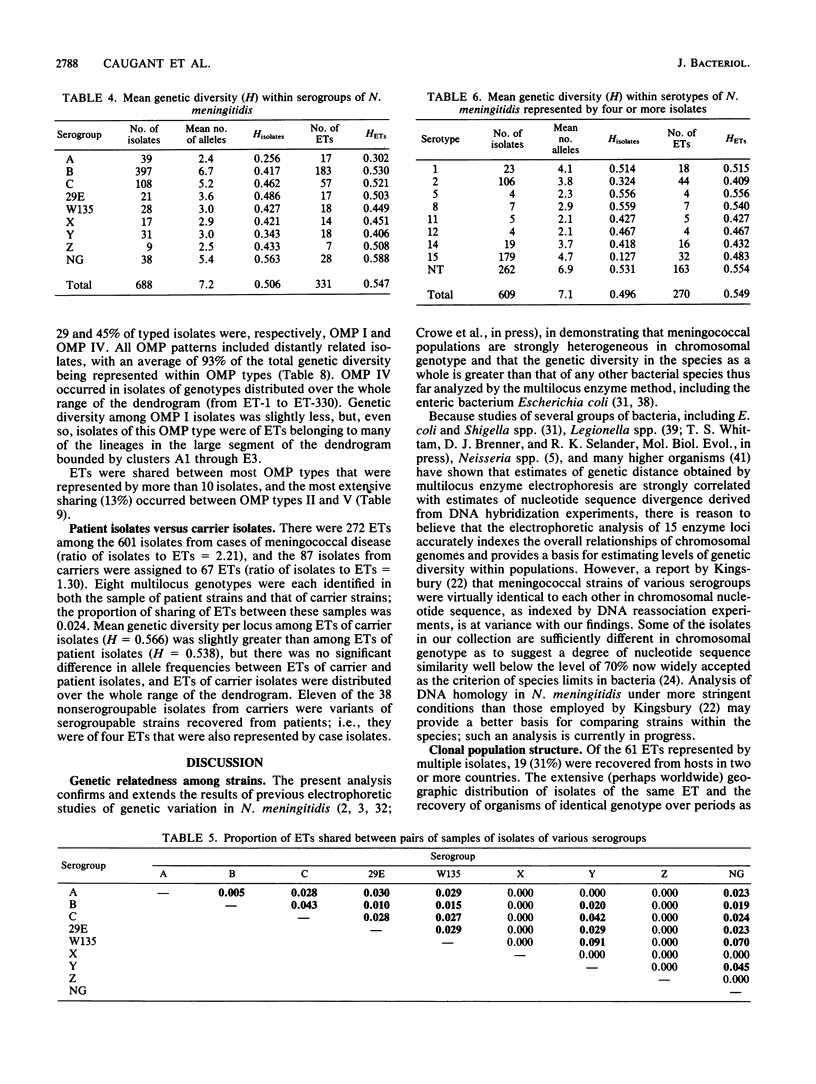
Abstract
The genetic structure of populations of Neisseria meningitidis was examined by an analysis of electrophoretically demonstrable allelic variation at 15 genes encoding enzymes in 650 isolates of eight serogroups (A, B, C, W135, X, Y, Z, and 29E) and 38 nonserogroupable isolates. A total of 331 distinctive multilocus genotypes (electrophoretic types, ETs) was identified, among which mean genetic diversity per locus (H = 0.547) was greater than in Escherichia coli and other bacterial species thus far studied. The intercontinental distribution of some ETs and the recovery of organisms of identical genotype over periods of many years strongly suggest that the genetic structure of N. meningitidis is basically clonal as a consequence of low rates of recombination of chromosomal genes. Variation among strains in serogroup, serotype, and the electrophoretic pattern of the major outer membrane proteins has little relationship to the complex structure of populations revealed by enzyme electrophoresis, which involves 14 major lineages of clones diverging from one another at genetic distances greater than 0.50. Genetic diversity among ETs of isolates of the same serogroup was, on average, 84% of that in the total sample. Clones of serogroup A were unusual in being genotypically less heterogeneous than those of other serogroups and in forming a single phylogenetic group. Isolates of the same serotype or outer membrane protein pattern were also highly heterogeneous; on average, 87 and 97%, respectively, of the total species diversity was represented by ETs of the same serotype or outer membrane protein.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Bøvre K., Gaustad P., Bryn K., Holten E., Høiby E. A., Frøholm L. O. Multilocus genotypes determined by enzyme electrophoresis of Neisseria meningitidis isolated from patients with systemic disease and from healthy carriers. J Gen Microbiol. 1986 Mar;132(3):641–652. doi: 10.1099/00221287-132-3-641. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Frøholm L. O., Bøvre K., Holten E., Frasch C. E., Mocca L. F., Zollinger W. D., Selander R. K. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4927–4931. doi: 10.1073/pnas.83.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Selander R. K. Genetic diversity and temporal variation in the E. coli population of a human host. Genetics. 1981 Jul;98(3):467–490. doi: 10.1093/genetics/98.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Zollinger W. D., Mocca L. F., Frasch C. E., Whittam T. S., Frøholm L. O., Selander R. K. Genetic relationships and clonal population structure of serotype 2 strains of Neisseria meningitidis. Infect Immun. 1987 Jun;55(6):1503–1512. doi: 10.1128/iai.55.6.1503-1512.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun P. K., Sensabaugh G. F., Vedros N. A. Genetic relationships among Neisseria species assessed by comparative enzyme electrophoresis. J Gen Microbiol. 1985 Nov;131(11):3105–3115. doi: 10.1099/00221287-131-11-3105. [DOI] [PubMed] [Google Scholar]
- Counts G. W., Seeley L., Beaty H. N. Identification of an epidemic strain of Neisseria meningitidis by bacteriocin typing. J Infect Dis. 1971 Jul;124(1):26–32. doi: 10.1093/infdis/124.1.26. [DOI] [PubMed] [Google Scholar]
- Craven D. E., Frasch C. E., Mocca L. F., Rose F. B., Gonzalez R. Rapid serogroup identification of Neisseria meningitidis by using antiserum agar: Prevalence of serotypes in a disease-free military population. J Clin Microbiol. 1979 Sep;10(3):302–307. doi: 10.1128/jcm.10.3.302-307.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven D. E., Peppler M. S., Frasch C. E., Mocca L. F., McGrath P. P., Washington G. Adherence of isolates of Neisseria meningitidis from patients and carriers to human buccal epithelial cells. J Infect Dis. 1980 Oct;142(4):556–568. doi: 10.1093/infdis/142.4.556. [DOI] [PubMed] [Google Scholar]
- DeVoe I. W. The meningococcus and mechanisms of pathogenicity. Microbiol Rev. 1982 Jun;46(2):162–190. doi: 10.1128/mr.46.2.162-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff T. C. In-vitro and in-vivo studies of resistance to rifampin in meningococci. J Infect Dis. 1971 Apr;123(4):414–420. doi: 10.1093/infdis/123.4.414. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., Chapman S. S. Classification of Neisseria meningitidis group B into distinct serotypes. 3. Application of a new bactericidal-inhibition technique to distribution of serotypes among cases and carriers. J Infect Dis. 1973 Feb;127(2):149–154. doi: 10.1093/infdis/127.2.149. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., Zollinger W. D., Poolman J. T. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev Infect Dis. 1985 Jul-Aug;7(4):504–510. doi: 10.1093/clinids/7.4.504. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Broud D. D., Silver C. A., Artenstein M. S. Immunoepidemiology of meningococcal disease in military recruits. I. A model for serogroup independency of epidemic potential as determined by serotyping. J Infect Dis. 1977 Aug;136(2):176–186. doi: 10.1093/infdis/136.2.176. [DOI] [PubMed] [Google Scholar]
- Holten E. Glucokinase and glucose 6-phosphate dehydrogenase in Neisseria. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Apr;82(2):201–206. doi: 10.1111/j.1699-0463.1974.tb02312.x. [DOI] [PubMed] [Google Scholar]
- Holten E., Jyssum K. Glutamate dehydrogenases in Neisseria meningitidis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Feb;81(1):43–48. doi: 10.1111/j.1699-0463.1973.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Jackson P., Thornley M. J., Thompson R. J. A study by high-resolution two-dimensional polyacrylamide gel electrophoresis of relationships between Neisseria gonorrhoeae and other bacteria. J Gen Microbiol. 1984 Dec;130(12):3189–3201. doi: 10.1099/00221287-130-12-3189. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C. W., Ashton F. E., Ryan J. A. The structure of the capsular polysaccharide obtained from a new serogroup (L) of Neisseria meningitidis. Carbohydr Res. 1983 Jan 16;112(1):105–111. doi: 10.1016/0008-6215(83)88270-6. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T. Deoxyribonucleic acid homologies among species of the genus Neisseria. J Bacteriol. 1967 Oct;94(4):870–874. doi: 10.1128/jb.94.4.870-874.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Fanning G. R., Johnson K. E., Brenner D. J. Thermal stability of interspecies Neisseria DNA duplexes. J Gen Microbiol. 1969 Feb;55(2):201–208. doi: 10.1099/00221287-55-2-201. [DOI] [PubMed] [Google Scholar]
- Kristiansen B. E., Bjorvatn B., Lund V., Lindqvist B., Holten E. Differentiation of B15 strains of Neisseria meningitidis by DNA restriction endonuclease fingerprinting. J Infect Dis. 1984 Nov;150(5):672–677. doi: 10.1093/infdis/150.5.672. [DOI] [PubMed] [Google Scholar]
- MILLAR J. W., SIESS E. E., FELDMAN H. A., SILVERMAN C., FRANK P. IN VIVO AND IN VITRO RESISTANCE TO SULFADIAZINE IN STRAINS OF NEISSERIA MENINGITIDIS. JAMA. 1963 Oct 12;186:139–141. doi: 10.1001/jama.1963.63710020008016. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Lipopolysaccharide serotyping of Neisseria meningitidis by hemagglutination inhibition. Infect Immun. 1977 May;16(2):471–475. doi: 10.1128/iai.16.2.471-475.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocca L. F., Frasch C. E. Sodium dodecyl sulfate-polyacrylamide gel typing system for characterization of Neisseria meningitidis isolates. J Clin Microbiol. 1982 Aug;16(2):240–244. doi: 10.1128/jcm.16.2.240-244.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulks M. H., Plaut A. G., Feldman H. A., Frangione B. IgA proteases of two distinct specificities are released by Neisseria meningitidis. J Exp Med. 1980 Nov 1;152(5):1442–1447. doi: 10.1084/jem.152.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Granoff D. M., Pattison P. E., Selander R. K. A population genetic framework for the study of invasive diseases caused by serotype b strains of Haemophilus influenzae. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5078–5082. doi: 10.1073/pnas.82.15.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H., Whittam T. S., Caugant D. A., Selander R. K. Enzyme polymorphism and genetic population structure in Escherichia coli and Shigella. J Gen Microbiol. 1983 Sep;129(9):2715–2726. doi: 10.1099/00221287-129-9-2715. [DOI] [PubMed] [Google Scholar]
- Peltola H. Meningococcal disease: still with us. Rev Infect Dis. 1983 Jan-Feb;5(1):71–91. doi: 10.1093/clinids/5.1.71. [DOI] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunochemical characterization of Neisseria meningitidis serotype antigens by immunodiffusion and SDS-polyacrylamide gel electrophoresis immunoperoxidase techniques and the distribution of serotypes among cases and carriers. J Gen Microbiol. 1980 Feb;116(2):465–473. doi: 10.1099/00221287-116-2-465. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Morton G. Adherence of Neisseria meningitidis to human epithelial cells. Infect Immun. 1981 Jan;31(1):430–435. doi: 10.1128/iai.31.1.430-435.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., McKinney R. M., Whittam T. S., Bibb W. F., Brenner D. J., Nolte F. S., Pattison P. E. Genetic structure of populations of Legionella pneumophila. J Bacteriol. 1985 Sep;163(3):1021–1037. doi: 10.1128/jb.163.3.1021-1037.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C. G., Ahlquist J. E. The phylogeny of the hominoid primates, as indicated by DNA-DNA hybridization. J Mol Evol. 1984;20(1):2–15. doi: 10.1007/BF02101980. [DOI] [PubMed] [Google Scholar]
- Sippel J. E., Quan A. Homogeneity of protein serotype antigens in Neisseria meningitidis group A. Infect Immun. 1977 May;16(2):623–627. doi: 10.1128/iai.16.2.623-627.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen B., Falk E. S., Wisløff-Nilsen E., Bjorvatn B., Kristiansen B. E. Multivariate analysis of Neisseria DNA restriction endonuclease patterns. J Gen Microbiol. 1985 Nov;131(11):3099–3104. doi: 10.1099/00221287-131-11-3099. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Type-specific antigens of group A Neisseria meningitidis: lipopolysaccharide and heat-modifiable outer membrane proteins. Infect Immun. 1980 May;28(2):451–458. doi: 10.1128/iai.28.2.451-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Moran E. E., Connelly H., Mandrell R. E., Brandt B. Monoclonal antibodies to serotype 2 and serotype 15 outer membrane proteins of Neisseria meningitidis and their use in serotyping. Infect Immun. 1984 Oct;46(1):260–266. doi: 10.1128/iai.46.1.260-266.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



