Abstract
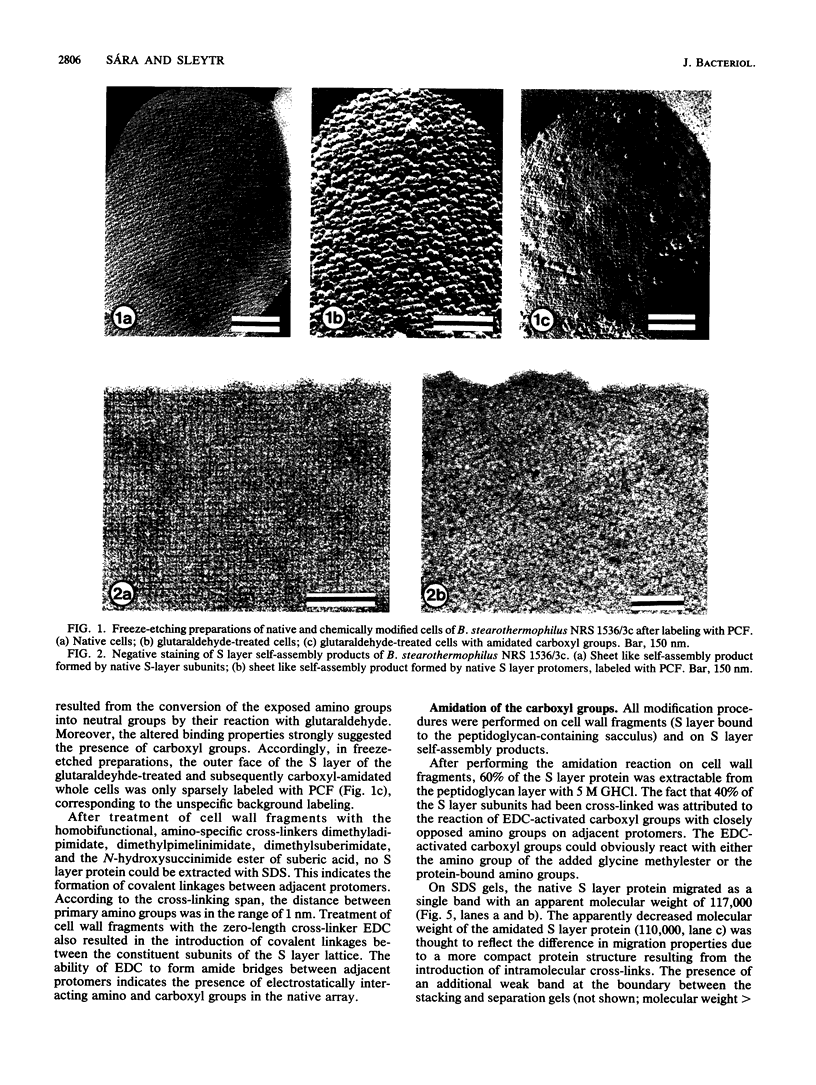
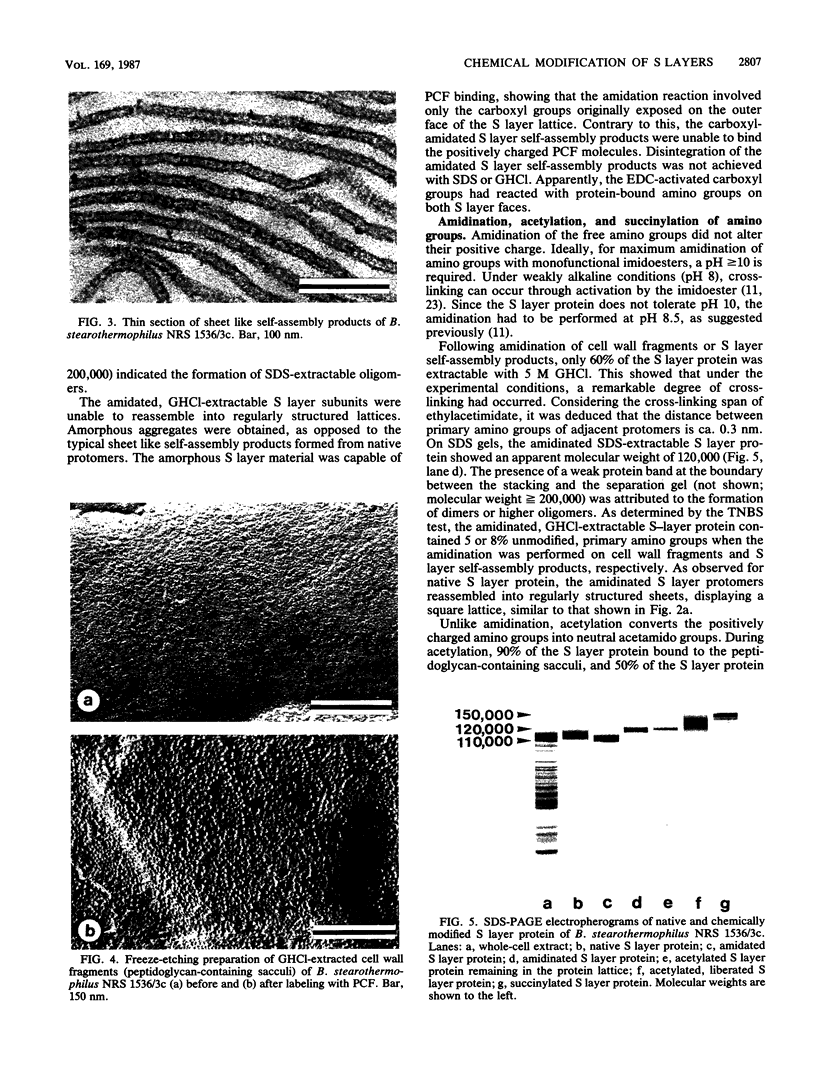
The distribution and functional significance of charged groups on the outer and inner faces of the S layer from Bacillus stearothermophilus NRS 1536/3c was investigated. Chemical modification of the exposed amino or carboxyl groups was performed on whole cells, isolated S layers self-assembled in vitro, and cell wall fragments (S layer attached to the peptidoglycan-containing sacculus). Without chemical modification, S layer self-assembly products could be labeled with polycationic ferritin, while S layers on whole cells could not. Following treatment with glutaraldehyde, whole cells were uniformly labeled with polycationic ferritin. Whole cells treated with glutaraldehyde and glycine methyl ester in the presence of carbodiimide did not bind polycationic ferritin significantly above background. Treatment of cell wall fragments with amino-specific, homobifunctional cross-linkers or with carbodiimide alone rendered the S layer protein nonextractable with sodium dodecyl sulfate. After amidation of the accessible carboxyl groups, the modified, guanidine hydrochloride-extractable S layer protomers did not self-assemble into regularly structured lattices. N-Amidination with ethylacetimidate did not interfere with the self-assembly of the isolated protomers. N-Acetylation resulted in a considerable destabilization of the S layer lattice, as seen by the release of a large amount of modified protomers during the reaction. N-Succinylation led to a complete disintegration of the protein lattice. These results indicated that only the inner face of the S layer carried a net negative charge. On both faces, free amino and carboxyl groups of adjacent protomers were arranged in proximity so as to contribute by electrostatic interactions to the cohesion of the protomers in the two-dimensional array. The native charge of the protomers was required for both the in vitro self-assembly of the isolated subunits and the maintenance of the structural integrity of the S layer lattice. Among other functions, the biological significance of the S layers may be in masking the electronegative charge of the cell wall proper.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Smith P. R., Dubochet J., Henry C., Kellenberger E. A study of the structure of the T-layer of Bacillus brevis. J Supramol Struct. 1973;1(6):498–522. doi: 10.1002/jss.400010606. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Dependence of the superficial layers of Spirillum putridiconchylium on Ca2+ or Sr2+. Can J Microbiol. 1976 Sep;22(9):1233–1244. doi: 10.1139/m76-183. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J. Surface arrays on the wall of Sporosarcina ureae. J Bacteriol. 1979 Sep;139(3):1039–1048. doi: 10.1128/jb.139.3.1039-1048.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- Galella G., Smith D. B. The cross-linking of tubulin with imidoesters. Can J Biochem. 1982 Jan;60(1):71–80. doi: 10.1139/o82-010. [DOI] [PubMed] [Google Scholar]
- Gounaris A. D., Perlmann G. E. Succinylation of pepsinogen. J Biol Chem. 1967 Jun 10;242(11):2739–2745. [PubMed] [Google Scholar]
- Hastie A. T., Brinton C. C., Jr Isolation, characterization, and in vitro assembly of the tetragonally arrayed layer of Bacillus sphaericus. J Bacteriol. 1979 Jun;138(3):999–1009. doi: 10.1128/jb.138.3.999-1009.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R., Osuga D. T., Feeney R. E. Modification of amino groups in inhibitors of proteolytic enzymes. Biochemistry. 1967 Feb;6(2):541–547. doi: 10.1021/bi00854a023. [DOI] [PubMed] [Google Scholar]
- Inman J. K., Perham R. N., DuBois G. C., Appella E. Amidination. Methods Enzymol. 1983;91:559–569. doi: 10.1016/s0076-6879(83)91051-0. [DOI] [PubMed] [Google Scholar]
- Ji T. H. Bifunctional reagents. Methods Enzymol. 1983;91:580–609. doi: 10.1016/s0076-6879(83)91053-4. [DOI] [PubMed] [Google Scholar]
- Kist M. L., Murray R. G. Components of the regular surface array of Aquaspirillum serpens MW5 and their assembly in vitro. J Bacteriol. 1984 Feb;157(2):599–606. doi: 10.1128/jb.157.2.599-606.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leduc M., Rousseau M., van Heijenoort J. Structure of the cell wall of Bacillus species C.I.P. 76-111. Eur J Biochem. 1977 Oct 17;80(1):153–163. doi: 10.1111/j.1432-1033.1977.tb11867.x. [DOI] [PubMed] [Google Scholar]
- Masuda K., Kawata T. Reassembly of a regularly arranged protein in the cell wall of Lactobacillus buchneri and its reattachment to cell walls: chemical modification studies. Microbiol Immunol. 1985;29(10):927–938. [PubMed] [Google Scholar]
- Masuda K., Kawata T. Reassembly of the regularly arranged subunits in the cell wall os Lactobacillus brevis and their reattachment to cell walls. Microbiol Immunol. 1980;24(4):299–308. doi: 10.1111/j.1348-0421.1980.tb02833.x. [DOI] [PubMed] [Google Scholar]
- Messner P., Pum D., Sára M., Stetter K. O., Sleytr U. B. Ultrastructure of the cell envelope of the archaebacteria Thermoproteus tenax and Thermoproteus neutrophilus. J Bacteriol. 1986 Jun;166(3):1046–1054. doi: 10.1128/jb.166.3.1046-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Rueckert R. R. Radiolabeling of proteins and viruses in vitro by acetylation with radioactive acetic anhydride. J Biol Chem. 1975 Feb 25;250(4):1413–1421. [PubMed] [Google Scholar]
- Perham R. N., Richards F. M. Reactivity and structural role of protein amino groups in tobacco mosaic virus. J Mol Biol. 1968 May 14;33(3):795–807. doi: 10.1016/0022-2836(68)90320-3. [DOI] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Glauert A. M. Ultrastructure of the cell walls of two closely related clostridia that possess different regular arrays of surface subunits. J Bacteriol. 1976 May;126(2):869–882. doi: 10.1128/jb.126.2.869-882.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Self-assembly of the hexagonally and tetragonally arranged subunits of bacterial surface layers and their reattachment to cell walls. J Ultrastruct Res. 1976 Jun;55(3):360–377. doi: 10.1016/s0022-5320(76)80093-7. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Sára M., Küpcü Z., Messner P. Structural and chemical characterization of S-layers of selected strains of Bacillus stearothermophilus and Desulfotomaculum nigrificans. Arch Microbiol. 1986 Oct;146(1):19–24. doi: 10.1007/BF00690152. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Thornley M. J., Naisbitt P., Glauert A. M. The nature of the attachment of a regularly arranged surface protein to the outer membrane of an Acinetobacter sp. Biochim Biophys Acta. 1975 Apr 21;389(1):97–116. doi: 10.1016/0005-2736(75)90388-0. [DOI] [PubMed] [Google Scholar]