Abstract
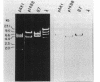
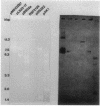
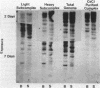
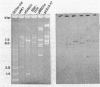
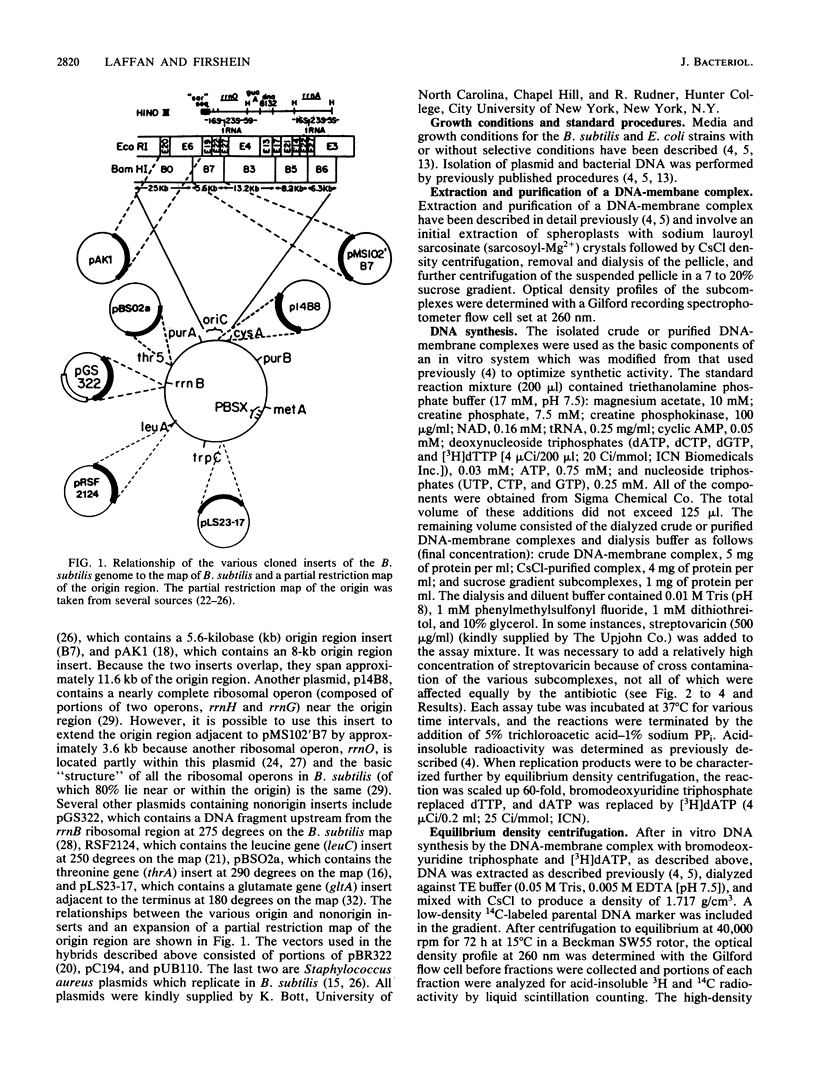
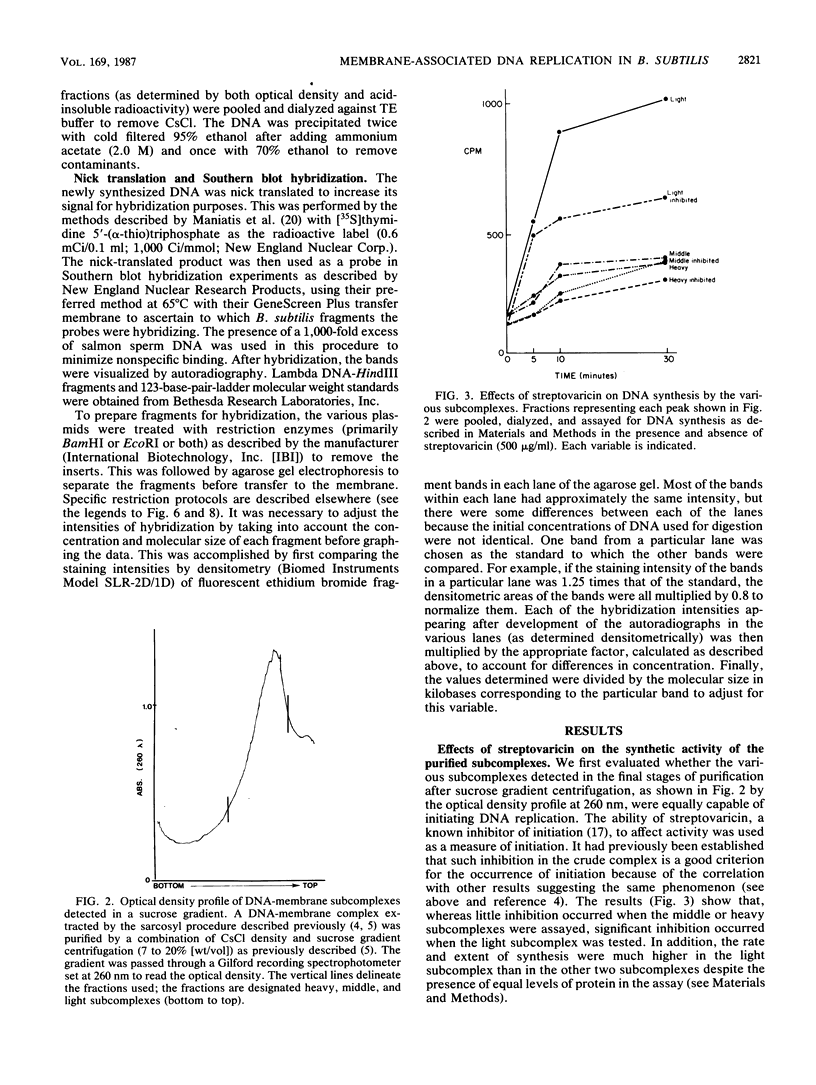
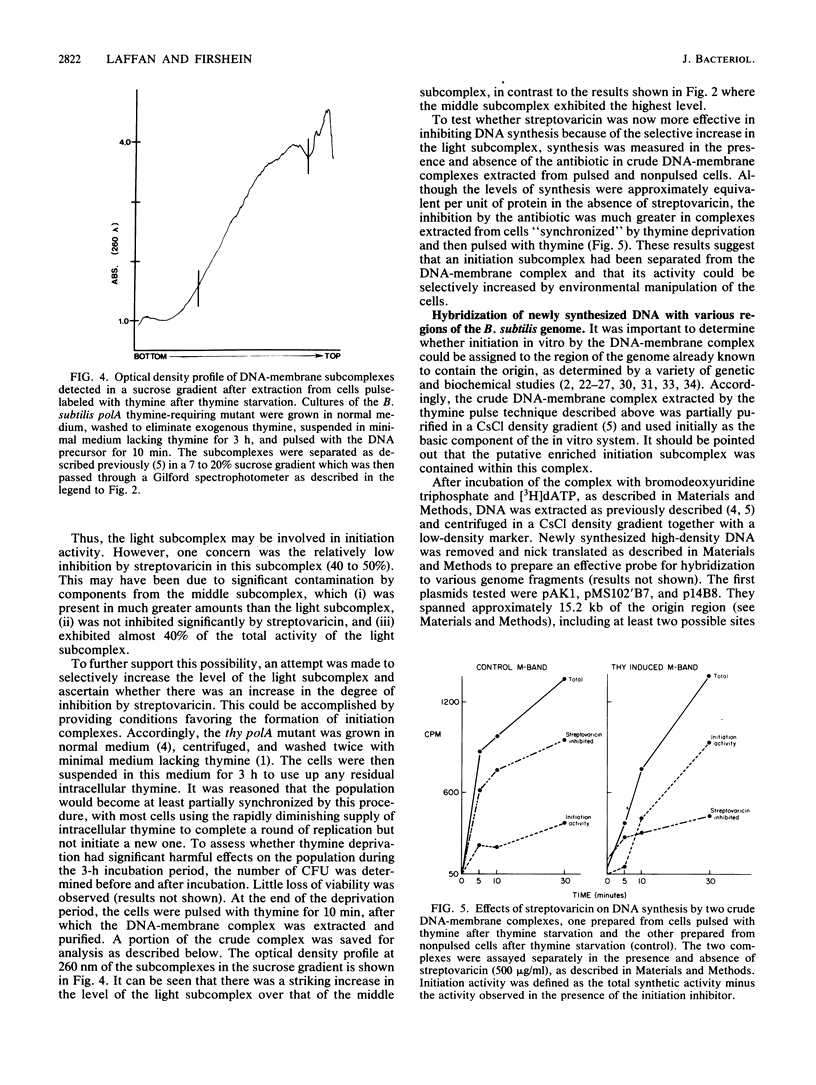
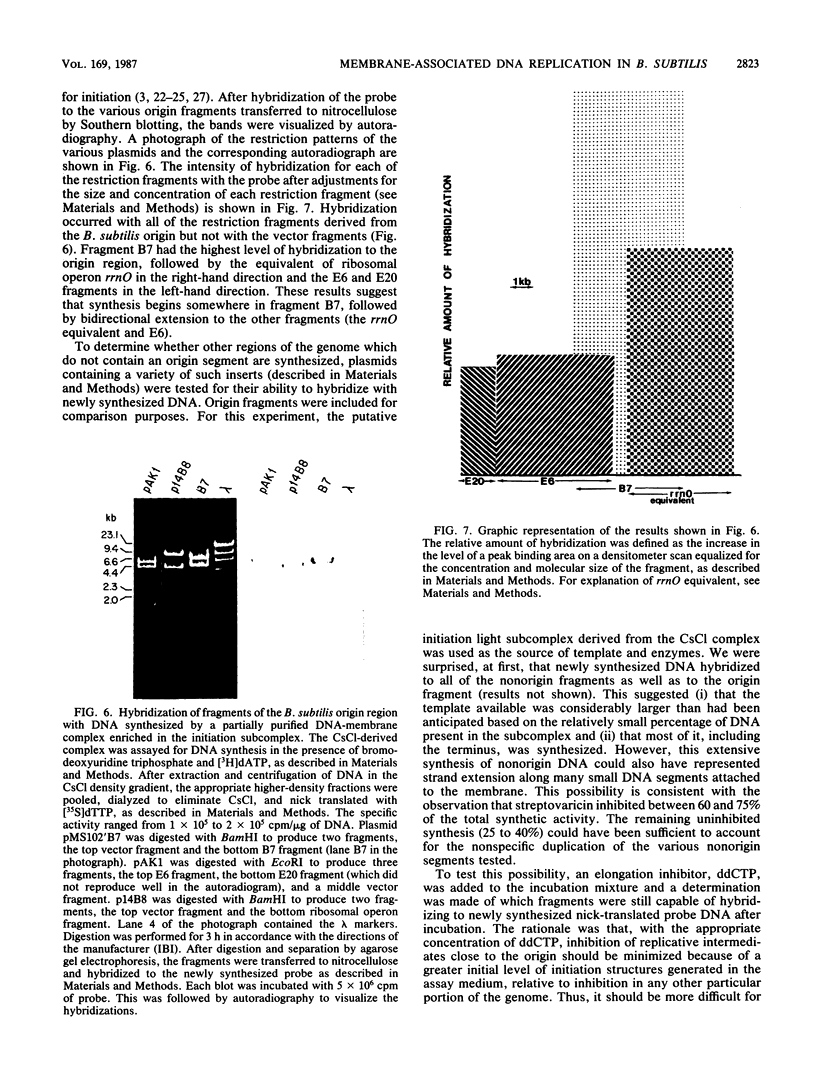
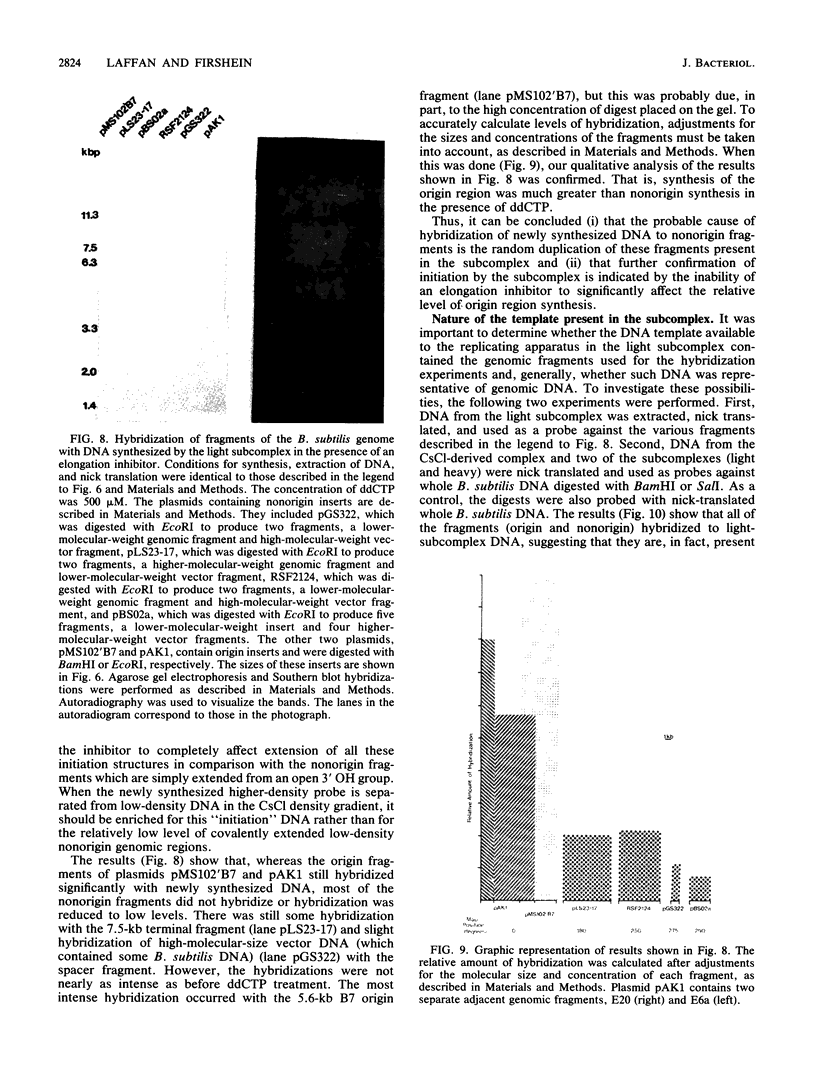
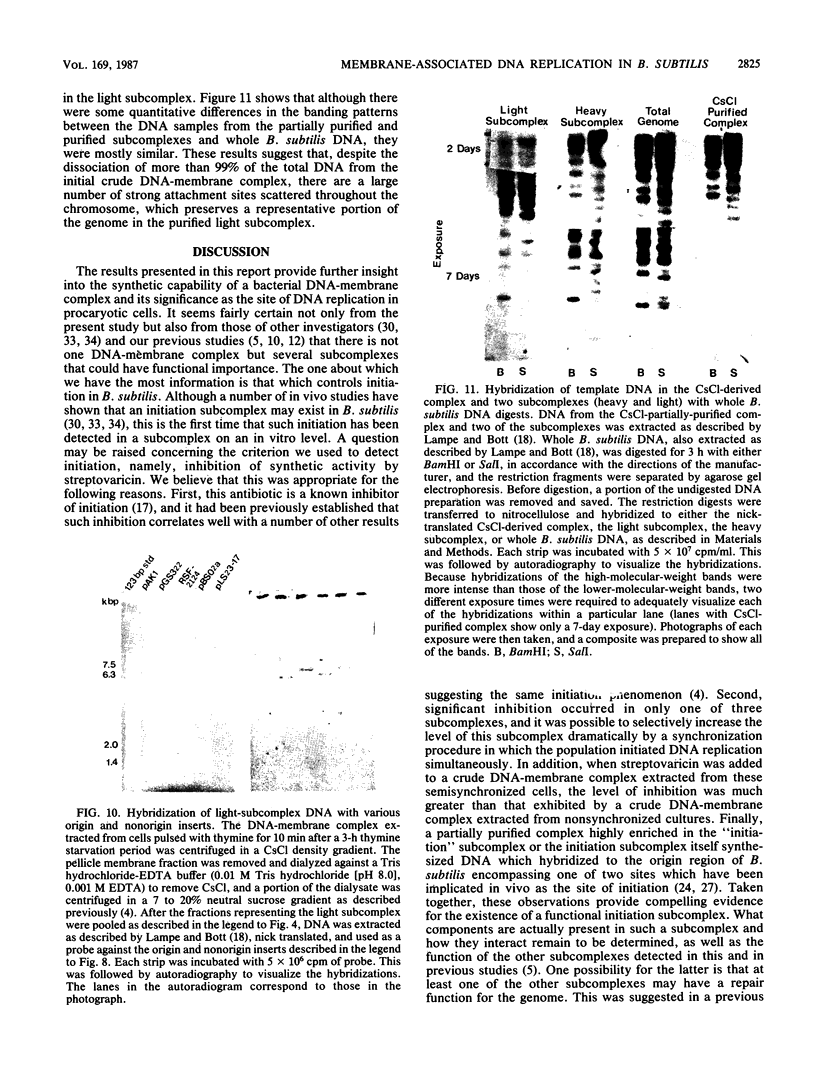
A DNA-membrane complex extracted from Bacillus subtilis was studied further as a model system for initiation of bacterial DNA replication in vitro. Of three subcomplexes purified from the crude complex by a combination of CsCl and sucrose gradient centrifugation, the synthetic capability of only one was inhibited significantly by streptovaricin, a known inhibitor of RNA primer formation. A selective enrichment in the level of this subcomplex was obtained by manipulating a thymine-requiring mutant. The synthetic capabilities of an enriched and nonenriched DNA-membrane complex were compared in the presence and absence of streptovaricin. Although the rate and extent of DNA synthesis per unit of protein were approximately the same in the absence of the antibiotic, there was a much greater inhibition of synthesis shown by the enriched complex in the presence of streptovaricin. Although the amount of DNA present in the putative initiation subcomplex was less than 0.3 to 0.4% of the total DNA present in the crude complex, such DNA, except for a few quantitative differences, was still representative of genomic DNA. Newly synthesized DNA hybridized to specific origin- and non-origin-derived restriction fragments of the B. subtilis genome. However, when an elongation inhibitor (ddCTP) was added, hybridization of such DNA to almost all of the nonorigin fragments disappeared or was reduced drastically, whereas origin region hybridization patterns remained strong. The highest level of hybridization in the origin region occurred with a BamHI (B7) restriction fragment of 5.6 kilobases that has been implicated by others as one site initiation in vivo (N. Ogasawara, M. Seiki, and H. Yoshikawa, Nature (London) 281:702-704, 1979; S. J. Seror-Laurent and G. Henckes, Proc. Natl. Acad. Sci. USA 82:3586-3590, 1985).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfalvi G., Sarkar N. Analysis of the 5'-termini of nascent DNA chains synthesized in permeable cells of Bacillus subtilis. J Mol Biol. 1983 Jan 15;163(2):147–169. doi: 10.1016/0022-2836(83)90001-3. [DOI] [PubMed] [Google Scholar]
- Beeson J., Sueoka N. Membrane enrichment of genetic markers close to the origin and terminus during the deoxyribonucleic acid replication cycle in Bacillus subtilis. J Bacteriol. 1979 Sep;139(3):911–916. doi: 10.1128/jb.139.3.911-916.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin P., Firshein W. Initiation of DNA replication in vitro by a DNA-membrane complex extracted from Bacillus subtilis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6214–6218. doi: 10.1073/pnas.80.20.6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin P., Strumph P., Kenny M., Firshein W. DNA synthesis in purified DNA-membrane complexes extracted from a Bacillus subtilis pol A mutant. Nature. 1982 Aug 19;298(5876):769–771. doi: 10.1038/298769a0. [DOI] [PubMed] [Google Scholar]
- Bezlepkin V. G., Malinovsky YuYu, Gaziev A. I. Formation of additional contacts of chromosome with membrane in the process of DNA repair synthesis in bacterial cells. Z Allg Mikrobiol. 1983;23(10):607–619. doi: 10.1002/jobm.3630231002. [DOI] [PubMed] [Google Scholar]
- Brown N. C. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J Mol Biol. 1971 Jul 14;59(1):1–16. doi: 10.1016/0022-2836(71)90409-8. [DOI] [PubMed] [Google Scholar]
- Firshein W., Caro L. Detection of displacement ("D") loops with the properties of a replicating intermediate synthesized by a DNA/membrane complex derived from the low-copy-number plasmid RK2. Plasmid. 1984 Nov;12(3):227–232. doi: 10.1016/0147-619x(84)90051-9. [DOI] [PubMed] [Google Scholar]
- Firshein W., Gelman I. W. Enrichment of DNA polymerase III activity in a DNA membrane complex purified from Pneumococcus: the possible existence of subcomplexes. Mol Gen Genet. 1981;182(1):87–94. doi: 10.1007/BF00422772. [DOI] [PubMed] [Google Scholar]
- Firshein W. In situ activity of enzymes on polyacrylamide gels of a deoxyribonucleic acid-membrane fraction extracted from pneumococci. J Bacteriol. 1974 Jun;118(3):1101–1110. doi: 10.1128/jb.118.3.1101-1110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firshein W., Strumph P., Benjamin P., Burnstein K., Kornacki J. Replication of a low-copy-number plasmid by a plasmid DNA-membrane complex extracted from minicells of Escherichia coli. J Bacteriol. 1982 Jun;150(3):1234–1243. doi: 10.1128/jb.150.3.1234-1243.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firshein W. The DNA-membrane fraction of Pneumococcus contains a DNA replication complex. J Mol Biol. 1972 Oct 14;70(3):383–397. doi: 10.1016/0022-2836(72)90547-5. [DOI] [PubMed] [Google Scholar]
- Firshein W. Two membrane sites for DNA synthesis in Pneumococcus. Mol Gen Genet. 1976 Nov 17;148(3):323–335. doi: 10.1007/BF00332907. [DOI] [PubMed] [Google Scholar]
- Greene M., Firshein W. Role of deoxyribonucleic acid ligase in a doxyribonucleic acid membrane fraction extracted from pneumococci. J Bacteriol. 1976 May;126(2):777–784. doi: 10.1128/jb.126.2.777-784.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe M. F., Bott K. F. Genetic and physical organization of the cloned gyrA and gyrB genes of Bacillus subtilis. J Bacteriol. 1985 Apr;162(1):78–84. doi: 10.1128/jb.162.1.78-84.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz P. J., Schaechter M. The attachment of the bacterial chromosome to the cell membrane. Int Rev Cytol. 1975;41:1–28. doi: 10.1016/s0074-7696(08)60964-x. [DOI] [PubMed] [Google Scholar]
- Nagahari K., Sakaguchi K. Cloning of Bacillus subtilis leucina A, B and C genes with Escherichia coli plasmids and expression of the leuC gene in E. coli. Mol Gen Genet. 1978 Jan 17;158(3):263–270. doi: 10.1007/BF00267197. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Mizumoto S., Yoshikawa H. Replication origin of the Bacillus subtilis chromosome determined by hybridization of the first-replicating DNA with cloned fragments from the replication origin region of the chromosome. Gene. 1984 Oct;30(1-3):173–182. doi: 10.1016/0378-1119(84)90118-5. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Seiki M., Yoshikawa H. Effect of novobiocin on initiation of DNA replication in Bacillus subtilis. Nature. 1979 Oct 25;281(5733):702–704. doi: 10.1038/281702a0. [DOI] [PubMed] [Google Scholar]
- Ogasawara N., Seiki M., Yoshikawa H. Replication origin region of Bacillus subtilis chromosome contains two rRNA operons. J Bacteriol. 1983 Apr;154(1):50–57. doi: 10.1128/jb.154.1.50-57.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Ogasawara N., Yoshikawa H. Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. I. Isolation and characterization of plasmids containing the origin region. Mol Gen Genet. 1981;183(2):220–226. doi: 10.1007/BF00270621. [DOI] [PubMed] [Google Scholar]
- Seiki M., Ogasawara N., Yoshikawa H. Structure of the region of the replication origin of the Bacillus subtilis chromosome. Nature. 1979 Oct 25;281(5733):699–701. doi: 10.1038/281699a0. [DOI] [PubMed] [Google Scholar]
- Stewart G. C., Bott K. F. DNA sequence of the tandem ribosomal RNA promoter for B. subtilis operon rrnB. Nucleic Acids Res. 1983 Sep 24;11(18):6289–6300. doi: 10.1093/nar/11.18.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. C., Wilson F. E., Bott K. F. Detailed physical mapping of the ribosomal RNA genes of Bacillus subtilis. Gene. 1982 Sep;19(2):153–162. doi: 10.1016/0378-1119(82)90001-4. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Hammers J. M. Isolation of DNA-membrane complex in Bacillus subtilis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4787–4791. doi: 10.1073/pnas.71.12.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Quinn W. G. Membrane attachment of the chromosome replication origin in Bacillus subtilis. Cold Spring Harb Symp Quant Biol. 1968;33:695–705. doi: 10.1101/sqb.1968.033.01.078. [DOI] [PubMed] [Google Scholar]
- Séror-Laurent S. J., Henckes G. An RNA-DNA copolymer whose synthesis is correlated with the transcriptional requirement for chromosomal initiation in Bacillus subtilis contains ribosomal RNA sequences. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3586–3590. doi: 10.1073/pnas.82.11.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. S., Wake R. G. Restriction map of DNA spanning the replication terminus of the Bacillus subtilis chromosome. J Mol Biol. 1983 Dec 5;171(2):119–137. doi: 10.1016/s0022-2836(83)80349-0. [DOI] [PubMed] [Google Scholar]
- Winston S., Sueoka N. DNA-membrane association is necessary for initiation of chromosomal and plasmid replication in Bacillus subtilis. Proc Natl Acad Sci U S A. 1980 May;77(5):2834–2838. doi: 10.1073/pnas.77.5.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]