Abstract
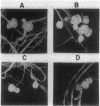
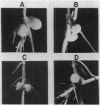
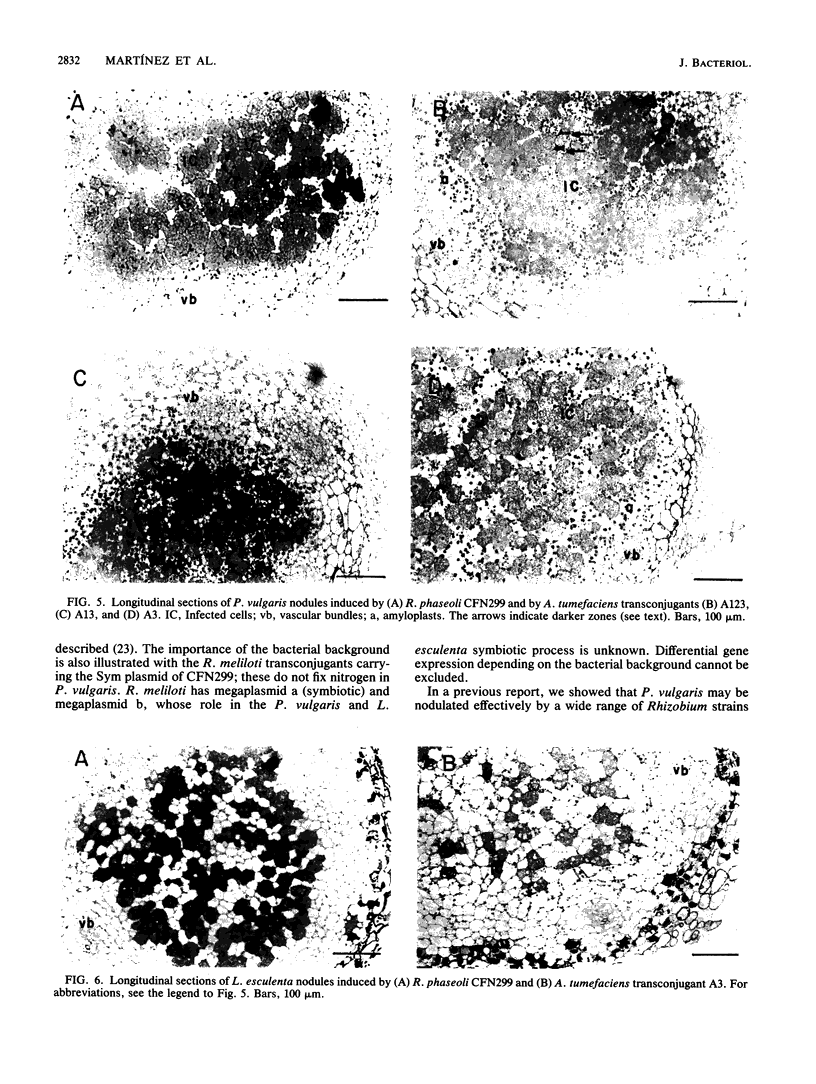
Rhizobium phaseoli CFN299 forms nitrogen-fixing nodules in Phaseolus vulgaris (bean) and in Leucaena esculenta. It has three plasmids of 185, 225, and 410 kilobases. The 410-kilobase plasmid contains the nitrogenase structural genes. We have transferred these plasmids to the plasmid-free strain Agrobacterium tumefaciens GMI9023. Transconjugants containing different combinations of the R. phaseoli plasmids were obtained, and they were exhaustively purified before nodulation was assayed. Only transconjugants harboring the 410-kilobase plasmid nodulate P. vulgaris and L. esculenta. Nodules formed by all such transconjugants are able to reduce acetylene. Transconjugants containing the whole set of plasmids from CFN299 nodulate better and fix more nitrogen than the transconjugants carrying only the Sym plasmid. Microscopic analysis of nodules induced by A. tumefaciens transconjugants reveals infected cells and vascular bundles. None of the A. tumefaciens transconjugants, not even the one with the whole set of plasmids from CFN299, behaves in symbiosis like the original R. phaseoli strain; the transconjugants produce fewer nodules and have lower acetylene reduction (25% as compared to the original R. phaseoli strain) and more amyloplasts per nodule. More than 2,000 bacterial isolates from nodules of P. vulgaris and L. esculenta formed by the transconjugants were analyzed by different criteria. Not a single rhizobium could be detected. Our results show that R. phaseoli plasmids may be expressed in the A. tumefaciens background and direct the formation of effective, differentiated nodules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Djordjevic M. A., Zurkowski W., Rolfe B. G. Plasmids and stability of symbiotic properties of Rhizobium trifolii. J Bacteriol. 1982 Aug;151(2):560–568. doi: 10.1128/jb.151.2.560-568.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic M. A., Zurkowski W., Shine J., Rolfe B. G. Sym plasmid transfer to various symbiotic mutants of Rhizobium trifolii, R. leguminosarum, and R. meliloti. J Bacteriol. 1983 Dec;156(3):1035–1045. doi: 10.1128/jb.156.3.1035-1045.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- FAHRAEUS G. The infection of clover root hairs by nodule bacteria studied by a simple glass slide technique. J Gen Microbiol. 1957 Apr;16(2):374–381. doi: 10.1099/00221287-16-2-374. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Hirsch A. M., Leigh J. A., Johansen E., Kuldau G. A., Deegan S., Walker G. C., Signer E. R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985 Apr;40(4):869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- Hamilton R. H., Fall M. Z. The loss of tumor-initiating ability in Agrobacterium tumefaciens by incubation at high temperature. Experientia. 1971 Feb 15;27(2):229–230. doi: 10.1007/BF02145913. [DOI] [PubMed] [Google Scholar]
- Hooykaas P. J., Snijdewint F. G., Schilperoort R. A. Identification of the Sym plasmid of Rhizobium leguminosarum strain 1001 and its transfer to and expression in other rhizobia and Agrobacterium tumefaciens. Plasmid. 1982 Jul;8(1):73–82. doi: 10.1016/0147-619x(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Hooykaas P. J., den Dulk-Ras H., Ooms G., Schilperoort R. A. Interactions between octopine and nopaline plasmids in Agrobacterium tumefaciens. J Bacteriol. 1980 Sep;143(3):1295–1306. doi: 10.1128/jb.143.3.1295-1306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooykaas P. J., den Dulk-Ras H., Regensburg-Tuïnk A. J., van Brussel A. A., Schilperoort R. A. Expression of a Rhizobium phaseoli Sym plasmid in R. trifolii and Agrobacterium tumefaciens: incompatibility with a R. trifolii Sym plasmid. Plasmid. 1985 Jul;14(1):47–52. doi: 10.1016/0147-619x(85)90031-9. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Sanchez A., Fernandez L., Leemans J., Cevallos M. A. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984 Apr;158(1):148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinto C., De La Vega H., Flores M., Leemans J., Cevallos M. A., Pardo M. A., Azpiroz R., De Lourdes Girard M., Calva E., Palacios R. Nitrogenase reductase: A functional multigene family in Rhizobium phaseoli. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1170–1174. doi: 10.1073/pnas.82.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., Leps W. T., Silver L. E., Brill W. J. Use of two-dimensional polyacrylamide gel electrophoresis to identify and classify Rhizobium strains. Appl Environ Microbiol. 1980 Feb;39(2):414–422. doi: 10.1128/aem.39.2.414-422.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. High frequency mobilization of gram-negative bacterial replicons by the in vitro constructed Tn5-Mob transposon. Mol Gen Genet. 1984;196(3):413–420. doi: 10.1007/BF00436188. [DOI] [PubMed] [Google Scholar]
- Soberón-Chávez G., Nájera R., Olivera H., Segovia L. Genetic rearrangements of a Rhizobium phaseoli symbiotic plasmid. J Bacteriol. 1986 Aug;167(2):487–491. doi: 10.1128/jb.167.2.487-491.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Truchet G., Rosenberg C., Vasse J., Julliot J. S., Camut S., Denarie J. Transfer of Rhizobium meliloti pSym genes into Agrobacterium tumefaciens: host-specific nodulation by atypical infection. J Bacteriol. 1984 Jan;157(1):134–142. doi: 10.1128/jb.157.1.134-142.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. H., Pankhurst C. E., Kondorosi A., Broughton W. J. Morphology of root nodules and nodule-like structures formed by Rhizobium and Agrobacterium strains containing a Rhizobium meliloti megaplasmid. J Cell Biol. 1983 Sep;97(3):787–794. doi: 10.1083/jcb.97.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]