Abstract
The insulin family of peptides and their receptors influence cellular growth in very early preimplantation embryos. In this study their expression and role in renal organogenesis was investigated. By immunofluorescence microscopy and in situ hybridization, insulin receptor (IR) expression was seen in the ureteric bud branches and early nephron precursors in mouse metanephroi harvested at day 13 of gestation. The expression gradually decreased in successive stages of gestation, and it was confined mainly to renal tubules in 1-week-old mice. Similar developmental regulation of the IR and insulin was observed by reverse transcriptase–polymerase chain reaction (RT-PCR) analyses. Addition of insulin into the culture medium at low concentrations, ranging from 40 to 400 ng/ml, induced trophic changes and increased [3H]thymidine incorporation in the embryonic renal explants, and inclusion of IR β-subunit-specific antisense oligodeoxynucleotide caused marked dysmorphogenesis and growth retardation of the metanephroi. Specificity of the antisense effect was reflected by immunoprecipitation experiments in which translational blockade of the β subunit of the IR was observed. RT-PCR analyses revealed that the α subunit of the IR was unaffected by the antisense treatment of metanephric explants. Concomitantly, de novo synthesis of morphogenetic regulatory extracellular matrix proteins, especially the proteoglycans, was decreased. Gel-shift analyses indicated a failure in the activation of c-fos promoter region binding protein(s) by insulin in the antisense oligodeoxynucleotide-treated explants. These studies suggest that insulin and its putative receptor are developmentally regulated in the murine embryonic metanephros, and they play a role in renal organogenesis, possibly by affecting other modulators of morphogenesis—i.e., extracellular matrix proteins and protooncogenes.
Keywords: kidney, development, growth factors/extracellular matrix, protooncogenes
Organogenesis in embryonic life constitutes a series of intricate processes which involve differentiation and rapid proliferation of pluripotent cells under the influence of a number of growth factors, including insulin and insulin-like growth factors (IGFs) (1–3). The IGFs and several other growth factors, upon binding to their putative transmembrane receptors, induce a multitude of effects—e.g., cell proliferation and induction of immediate-early genes (IEGs), and thus play a vital role in the development of various organs (3–5). However, the role of insulin and its receptor in embryonic development has received limited attention, which in part may be related to the fact that insulin and its receptor are primarily known for their regulation of glucose metabolism rather than as growth modulators (6). Nonetheless, the binding of insulin to the preimplantation mouse embryos, and expression of insulin receptor (IR) in the prepancreatic/precardiac stage of avian embryos and in early mid-gestational human fetuses raises the possibility for the growth potential of insulin (7–10). Furthermore, the expression of insulin during the prepancreatic stage at sites other than the pancreas—i.e., liver and yolk sac—suggests that insulin may play a role in mammalian embryonic development (11, 12). In support of this postulate are the studies in which insulin was shown to induce a proliferative response in avian embryonic myocytes and neuronal cells, and in the blastocysts and cells derived from 8- to 10-day-old whole mouse embryos (9, 13–15). This idea is further strengthened by studies in which target mutations in the insulin receptor gene resulted in the growth retardation of Drosophila and neonatal lethality in mice (16, 17). Although these studies suggest a potential role of insulin and its receptor in embryogenesis, their definitive role in the development of individual organ systems—e.g., the metanephros—has not been established.
Metanephric development ensues by the interaction of the ureteric epithelial bud with loosely organized mesenchyme, leading to the formation of an epithelial condensate that undergoes comma- and S-shaped-body and precapillary stages; the latter is vascularized and matures into a functioning nephron (18–20). These epithelial–mesenchymal interactions are heavily influenced by protooncogenes, growth factors, and extracellular matrix (ECM) proteins (21). The latter include some of the known regulators of morphogenesis—e.g., type IV collagen, laminin, and proteoglycans (19, 22). In view of the above considerations, studies were initiated to investigate the role of insulin and its receptor in mammalian metanephric development, and to establish the relevance of some of the morphogenetic ECM proteins and IEGs—i.e., c-fos—in renal organogenesis.
MATERIALS AND METHODS
Animals.
Paired mating of ICR mice (Harlan Sprague–Dawley) was carried out, and kidneys were harvested from fetuses at days 13, 17, and 19 (newborn) of gestation, and from 1-week-old mice.
Expression of IR.
For immunohistochemistry (23), 4-μm-thick cryostat sections of the kidneys were incubated successively with rabbit anti-IR polyclonal antibody and fluorescein isothiocyanate-conjugated anti-rabbit IgG (Upstate Biotechnology, Lake Placid, NY), and examined with an ultraviolet microscope equipped with epi-illumination. For in situ hybridization (23), 3-μm-thick tissue sections were prepared from the hilus of the paraformaldehyde-fixed and paraffin-embedded kidneys. After deparaffinization, hydration, and deproteination of the sections, they were then hybridized with adenosine 5′-[γ-[35S]thio]triphosphate (ATP[γ-35S])-labeled antisense oligodeoxynucleotide (ODN; 5′-GCCCCGCTCCAGGGCAAAATGCTTCCG-3′), the sequence of which was derived from upstream of the ATP-binding domain of the β subunit of mouse IR (ref. 24; see Fig. 6). Control sections were hybridized with sense ODN. The sections were washed with 0.15 M NaCl/0.015 M sodium citrate, dehydrated, and coated with NTB-2 emulsion (Kodak). Tissue autoradiograms were prepared after 2–3 weeks of exposure and examined with a microscope equipped with dark-field illumination. For reverse transcriptase (RT)-PCR analyses (23), total RNA was extracted from kidneys by the guanidinium thiocyanate/CsCl gradient centrifugation method (25). An exact amount of RNA—i.e., 10 μg, was used for oligo(dT)-primed first-strand cDNA synthesis.§ The cDNA was dissolved in 10 μl of deionized water, and 2 μl of cDNA was amplified using a 5′ primer (5′-GCGAAGATCCCTTGAAGAGGTGGG-3′) and a 3′ primer (5′-GCCCCGCTCCAGGGCAAAATGCTTCCG-3′) (24) and their primer designations were IR-I and IR-II, respectively (see Fig. 6). The IR-II primer had a sequence identical to the antisense ODN used for in situ hybridization (see above). The cDNA amplification was performed in a Thermal Cycler (Perkin–Elmer) as previously detailed (23). Each amplification cycle consisted of denaturation for 3 min at 94°C, annealing at 60°C for 2 min, and extension for 2 min at 72°C, and a total of 20 thermal cycles were employed. The resulting PCR product was examined by 1% agarose gel electrophoresis, and its identity was confirmed by cloning it into a pCR II plasmid vector (Invitrogen) followed by nucleotide sequence analysis (23). As a control, amplification of β-actin was also performed by using the same amount of cDNA as employed for the amplification of IR cDNA.
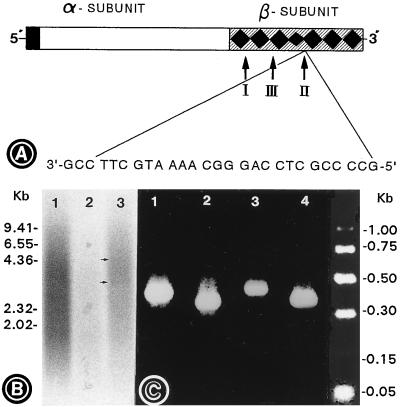
Figure 6.
(A) Locations of various primers in the β subunit of the proinsulin receptor gene. The oligodeoxynucleotide primers I, II, and III are the designations for insulin receptors I (IR-I), II (IR-II), and III (IR-III) as described in the text. IR-I is a sense primer, while IR-II and IR-III are antisense primers. Primer II was used as an antisense ODN in all the metanephric culture experiments, and its nucleotide sequence was derived upstream from the ATP-binding site. (B) Autoradiogram of the first-strand cDNAs prepared from untreated (lane 1) and antisense-treated (lanes 2 and 3) explants. The cDNA in lane 1 was synthesized using oligo(dT) as the primer. The cDNAs in lanes 2 and 3 were synthesized using IR-II and IR-III as the primers, respectively. A marked decrease in the synthesis of first-strand cDNA is observed when IR-II primer and mRNA from antisense-treated explants were used, as compared with the control (lane 2 vs. lane 1). The cDNA prepared by using antisense-treated explants and IR-III primer had faint bands (arrows in lane 3), corresponding to the size of intact proinsulin and its α subunit. (C) Agarose gel electrophoretograms of PCR products generated by using either IR-I and IR-II (lanes 1 and 3) or IR-I and IR-III (lanes 2 and 4) as primer sets, and cDNAs of untreated (lanes 1 and 2) and antisense-ODN-treated (lanes 3 and 4) explants. A notable decrease in band intensity is observed when cDNA of antisense-ODN-treated explants and the IR-I and -II primer set were used.
Expression of Insulin.
Studies were initiated to investigate the developmental regulation of its ligand—i.e., insulin—in embryonic and neonatal kidneys by RT-PCR. The respective 5′ and 3′ primers for the proinsulin-I gene were 5′-GTTGGTGCACTTCCTACCCCTG-3′ and 5′-GTAGAGGGAGCAGATGCTGGTG-3′, and those for the proinsulin-II gene were 5′-GTGGATGCGCTTCCTGCCCCTG-3′ and 5′- GTAGAGGGAGCAGATGCTGGTG-3′ (26). The PCR-based cDNA amplification conditions were the same as described above except for an increase in the annealing temperature from 60°C to 64°C and thermal cycles from 20 to 30. The identity of the amplified PCR products was established by nucleotide sequence analyses.
Effect of Insulin on Metanephric Development.
Metanephroi were harvested at day 13 of gestation and maintained in an organ culture system (27) in the presence or absence of an insulin mixture. The mixture included immediate-onset–short-acting and delayed-onset–long-acting insulin preparations (Eli Lilly). They were used at a concentration range of 1–10 milliunits/ml (40–400 ng/ml) and were added into the medium daily. After 4 days of insulin exposure, the explants were processed for morphological studies (23). In a separate experiment, explants were radiolabeled with [3H]thymidine for 12 hr prior to termination of the culture and were processed for tissue autoradiography (23).
Antisense Experiments.
A 27-mer phosphorothioated antisense ODN with IR-II primer nucleotide sequences, upstream of the tyrosine kinase domain of the β subunit of mouse IR, was synthesized. The phosphorothioated antisense ODN was included in the metanephric culture medium at 0.1–1.0 μM along with insulin (7.5 milliunits/ml) as described above. This antisense ODN exhibited no significant homology with known mammalian nucleotide sequences available in the GenBank EMBL database, and its specificity for the target nucleotide sequences was established by S1 nuclease protection assay (23). The controls included sense ODN and two additional 31-mer phosphorothioated ODNs with nonsense sequences—i.e., 5′-TAATGATAGTAATGATAGTAATGATAGTAAT-3′ and 5′-GATCGATCGATCGATCGATCGATCGATCGAT-3′. The explants were individually exposed to sense and antisense ODNs in the presence/absence of insulin for 4 days in culture, and were processed for morphological studies (23). To assess the specificity of antisense ODN, immunofluorescence and immunoprecipitation studies were performed, using rabbit anti-IR antibody as described above. For immunoprecipitation, sense- and antisense-ODN-treated explants were radiolabeled with [35S]methionine (250 μCi/ml; Amersham; 1 μCi = 37 kBq) for 12 hr prior to termination of the culture. The labeled explants were extracted with immunoprecipitation buffer (50 mM Tris⋅HCl, pH 7.4/50 mM NaCl/5 mM EDTA/1% Triton X-100) in the presence of protease inhibitors. Aliquots of the extracts, containing equal amounts of radiolabeled proteins from each variable, were used for immunoprecipitation followed by SDS/10% PAGE and autoradiography (23).
The specific cleavage of the targeted gene—i.e., the β subunit of the IR, was also determined by RT-PCR. Initially, synthesis of first-strand cDNA, from equal amounts of total RNA of 24-hr-treated explants, was carried out using either IR-II primer or another primer, designated as IR-III, whose nucleotide sequence was derived upstream from the antisense IR-II primer (see Fig. 6). The IR-III primer had the following sequence: 5′-CAGCTCCTCATCACCATATCGGC-3′. The control included the synthesis of first-strand cDNA, from an equal amount of RNA of “untreated” explants, using oligo(dT) as the primer. [α-32P]dCTP was included in the reaction mixture to monitor the cDNA synthesis in each of the three variables. An aliquot of [α-32P]dCTP-labeled cDNA was subjected to 1% agarose gel electrophoresis, followed by autoradiography. The three first-strand cDNAs, synthesized by priming with oligo(dT), IR-II, or IR-III, were purified by extraction with phenol/chloroform and precipitation with ethanol in the presence of glycogen (10 μg/ml), and then employed for PCR analyses. For PCR, sets of either IR-I and IR-II or IR-I and IR-III primers were used with expected product sizes of ≈0.45 and ≈0.4 kb, respectively. The PCR products were subjected to 2% agarose gel electrophoresis, and densitometric readings were made on the Polaroid negatives to determine the intensity of the bands (23). The identity of the bands was confirmed by subcloning the PCR products followed by nucleotide sequencing.
Expression of ECM Proteins.
The status of major ECM morphogenetic regulatory proteins (22), including perlecan, type IV collagen, and laminin, was examined. Embryonic kidneys, harvested at day 13 of gestation, were exposed to 0.5 μM antisense or sense ODN and radiolabeled with [35S]methionine for 12 hr prior to termination of the organ culture. The metanephric explants were extracted with 6 M guanidine⋅HCl in the presence of protease inhibitors (23). Aliquots containing equal amounts of incorporated radioactivity from each variable were processed for immunoprecipitation of ECM proteins with specific antibodies (23). The immunoprecipitated products were subjected either to SDS/polyacrylamide (5% acrylamide for laminin and collagen) or SDS/polyacrylamide/agarose (1.2% acrylamide + 0.6% agarose for proteoglycan) gel electrophoresis (23). The gels were dried and autoradiograms were prepared.
DNA–Protein Binding and Gel-Mobility Assays.
These experiments were performed to explore possible mechanism(s) responsible for the accelerated growth of the metanephros after stimulation by insulin. It is conceivable that IEGs were induced with the exposure of insulin in a manner similar to that for other growth factors that cause mitogenic stimuli in various biological systems (5). The best-characterized members of the IEGs include c-fos, c-jun, and Egr-1, and studies were targeted to the promoter region of c-fos.
First, nuclear extracts were prepared from embryonic kidneys treated with insulin for 30 min in presence or absence of IR antisense ODN in culture by following the method of Dignam et al. (28). Aliquots of each variable were made after adjusting the final protein concentration to 1 μg/μl and stored at −80°C until further use.
A double-stranded ODN (dsODN) with sequences representing the sis inducible element (SIE) of c-fos promoter (i.e., 5′-GTGCATTTCCCGTAAATCTTGTCTACAATTC-3′) (29) was synthesized and radiolabeled with ATP[γ-35S] (Amersham) using T4 polynucleotide kinase. The binding reaction mixture included 1:10 diluted Hepes buffer (10 mM Hepes, pH 7.9/1.2 M NaCl/10 mM MgCl2/10 mM dithiothreitol/1 mM EDTA), dsODN (10,000 cpm), 5 μg of poly[d(I-C)], 10 μg of nuclear protein extract, and bovine serum albumin with a final concentration of 300 μg/ml. The reaction was carried out at 30°C for 15 min. Specificity of the binding was ascertained by adding a 10-fold excess of unlabeled dsODN to competitively inhibit DNA–protein interactions. In a separate experiment, anti-phosphotyrosine antibody (Upstate Biotechnology) was added to the reaction mixture to determine if the binding protein is phosphorylated at the tyrosine residues. The binding complexes of various reaction mixtures were subjected to native (nondenaturing) 5% PAGE at 4°C. The gels were dried and autoradiograms were prepared.
RESULTS
Expression of IR.
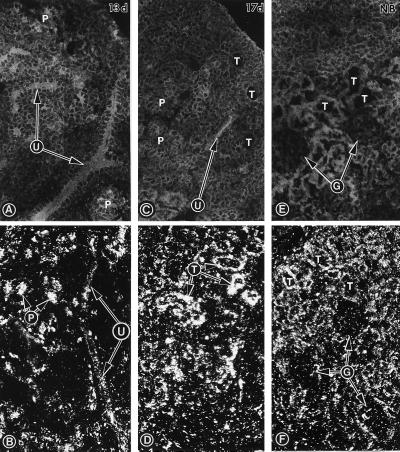
At day 13 of gestation, immunofluorescence microscopy revealed the expression of IR confined to the plasmalemma of cells of the ureteric bud branches and precursors of nascent nephrons representing early developmental stages—i.e, condensate, comma-, and S-shaped bodies (Fig. 1A). A similar expression was observed at day 17, and, in addition, it was also seen in the epithelia of maturing tubules (Fig. 1C). At day 19 or in newborns, the IR expression was restricted to the tubular epithelia, and it was absent from mature glomeruli (Fig. 1E).
Figure 1.
Immunofluorescence micrographs (A, C, and E) and dark-field illumination tissue autoradiograms (B, D, and F) showing expression of IR in metanephroi harvested at days 13 (A and B), 17 (C and D), and 19 (newborn, NB; E and F) of gestation. The immunofluorescence micrographs were prepared by staining the sections with a polyclonal anti-IR antibody. The autoradiograms were prepared by hybridizing the tissue sections in situ with [35S]ATP-labeled antisense ODN specific for IR. At day 13, IR message is seen on the ureteric bud branches (U) and precursors (P) of nascent nephrons. At day 17, the expression on the ureteric bud branches is reduced, while a high level of message is seen in the tubules (T; D). At day 19 (newborn), a mild decrease in the message on the tubules is observed, while no expression is seen in the glomeruli (G). (×50.)
In situ hybridization with ATP[γ-35S]-labeled ODN, derived from nucleotide sequence upstream from the tyrosine kinase domain of IR, revealed a spatiotemporal RNA expression similar to that observed in immunohistochemical studies. The IR mRNA expression was confined to the ureteric bud branches and early precursors of nascent nephrons at day 13 (Fig. 1B), and it became accentuated in tubular segments of the metanephros by day 17 (Fig. 1D). At day 19 or in the newborn, the IR mRNA expression was decreased but persisted in the tubules, and it was remarkably reduced in the mature glomeruli (Fig. 1F). A further decrease in IR mRNA was observed in the tubular epithelia of 1-week-old mouse kidneys.
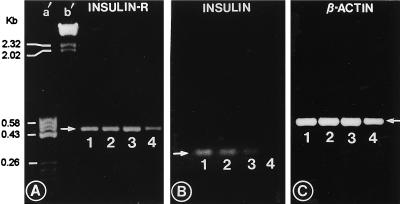
By RT-PCR, utilizing IR-I and IR-II primers (see Fig. 6), the mRNA expression of insulin receptor in the whole metanephros was found to be similar at days 13 and 17 of gestation and in the newborn mice (Fig. 2A). However, the expression was notably reduced in kidneys of 1 week-old mice. The analyses of electrophoretic bands of various PCR products revealed that they had identical nucleotide sequence to that reported in the literature for insulin receptor (24). The mRNA expression of β-actin in kidneys remained constant throughout the murine embryonic development and postnatal life (Fig. 2C).
Figure 2.
Agarose gel electrophoretograms of PCR products depicting the expression of insulin-proreceptor (A), preproinsulin (B), and β-actin (C) in kidneys harvested at days 13 (lane 1), 17 (lane 2), and 19 (lane 3) of gestation and from 1-week-old mice (lane 4). Lanes a′ and b′ represent the molecular weight markers. Expression of both the insulin and its receptor is seen at day 13, and it gradually diminishes in successive stages of development extending into the postnatal period, whereas the expression of β-actin remains constant during fetal life.
Expression of Insulin Genes.
The message for both proinsulin-I and proinsulin-II was detectable in the cDNAs of embryonic kidneys; however, a consistent amplification of the proinsulin-II gene could not be achieved by RT-PCR. Nonetheless, analyses of both the PCR products showed nucleotide sequences identical to proinsulin-I and -II genes. The message for the proinsulin-I gene was consistently detectable in the metanephric explants harvested during embryonic and postnatal period, and an expected band of ≈0.3 kb was observed by agarose gel electrophoresis (Fig. 2B). The highest expression of proinsulin-I was observed at day 13, and it was reduced by day 17 of gestation. A further notable reduction in the expression of proinsulin-I mRNA was observed in kidneys at day 19, while no message was detected in kidneys of 1-week-old mice, suggesting that its expression is also developmentally regulated like that of the insulin receptor.
Role of Insulin and Its Receptor in Metanephric Development.
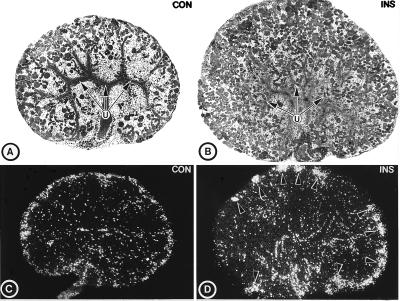
In these studies the effect of insulin and translational blockade of its receptor was ascertained. Inclusion of insulin (1–10 milliunits/ml) in the culture medium induced a dose-dependent increase in the size of metanephroi. The increase was discernible at a concentration of 2.5 milliunits/ml (100 ng/ml or 1.7 × 10−8 M), and the maximal effect was observed at 7.5 milliunits/ml (Fig. 3 B vs. A). Besides hypertrophy of the metanephros, the population of the nascent nephrons seemed to be increased as well (Fig. 3B). This insulin-induced hyperplastic response in the metanephros was reflected by an increase in [3H]thymidine-associated radioactivity, as observed in the tissue autoradiograms (Fig. 3 D vs. C). The increase in the incorporated radioactivity was noted especially in the cortical nephrogenic zone of the metanephros, the region where nascent nephrons are formed (Fig. 3D, arrowheads).
Figure 3.
Light micrographs (A and B) and dark-field tissue autoradiograms (C and D) of untreated (A and C) and insulin (7.5 milliunits/ml)-treated (B and D) metanephric explants, harvested at day 13 of gestation. The autoradiograms were prepared from explants radiolabeled with [3H]thymidine. The insulin-treated explants are large and have increased population of nephrons (B) and increased radioincorporation in the nephrogenic zone (arrowheads in D) as compared with the control. U, ureteric bud branches. (×20.)
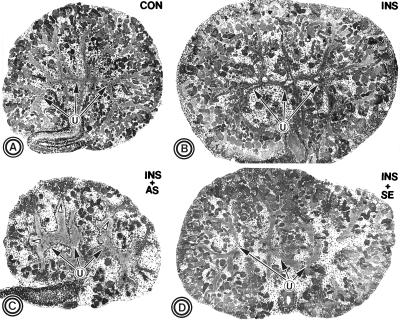
Kidneys treated with IR antisense ODN, IR-II, exhibited a retardation in their growth compared with those exposed to insulin (Fig. 4 C vs. B). The metanephroi were smaller than the untreated control explants as well (Fig. 4 C vs. A). Interestingly, the nephron population was remarkably reduced, and dysmorphogenesis of the ureteric bud branching was observed (Fig. 4C). The ureteric bud branches were swollen and disorganized, and their tips, the site where nephrogenesis ensues, were blunted (Fig. 4C, arrowheads). The metanephroi treated with sense or nonsense ODNs showed minimal changes, and insulin-induced hypertrophic/hyperplastic response in them was quite evident (Fig. 4D), suggesting that the effects of IR antisense ODN were specific.
Figure 4.
Light micrographs of the metanephric explants untreated (A) or treated with insulin (B), insulin + IR antisense ODN (C), and insulin + IR sense ODN (D). A remarkable reduction in the size and population of nephrons, along with disorganization of the ureteric bud branches (U) and blunting of their tips (arrowheads) is observed in explants treated with antisense ODN (C). A represents an untreated explant, while D shows the morphology of another control explant treated with sense ODN. (×20.)
Translational Blockade and Cleavage of mRNA of IR.
The specificity of the IR-II antisense ODN-induced morphologic changes was confirmed by translational blockade experiments. The metanephroi exposed to IR-II antisense ODN had diminished immunoreactivity with anti-IR antibody (Fig. 5 B vs. A). Similarly, a marked reduction in the radioactivity associated with the 95-kDa band of β subunit of IR was observed in [35S]methionine-labeled explants treated with IR-II antisense ODN (Fig. 5C, lane 3 vs. lanes 1 and 2). Interestingly, the 135-kDa band of the α subunit of IR was relatively less affected by the antisense-ODN treatment. This selective effect of the antisense ODN was further investigated by the RT-PCR experiments performed along the various nucleotide stretches of the IR β subunit.
Figure 5.
(A and B) Immunofluorescence micrographs of the explants treated with sense (A) and antisense (B) ODN and stained with anti-IR antibody. (×40.) A remarkable loss of antibody immunoreactivity is observed with the antisense-ODN treatment. U, ureteric bud branches. (C) SDS/PAGE autoradiogram of the [35S]methionine-labeled untreated control (CON), sense (SE), and antisense (AS)-treated explants, the extracts of which were immunoprecipitated with anti-IR antibody. In the control (lane 1), two bands (arrows) of radioactivity, corresponding to α and β subunits of IR, are seen. Antisense-ODN treatment resulted in a mild reduction of the immunoprecipitated radioactivity associated with the α subunit of the IR (upper band in lane 3 of C), whereas there is a drastic reduction in the radioactivity associated with β subunit, and the lower band is not discernible in the SDS/PAGE autoradiogram (lane 3 of C).
In the untreated control, the first-strand cDNA, generated by using oligo(dT) primer, had incorporated radioactivity = 5.59 × 107 cpm/μg of RNA, and a diffuse smear of radioactivity was seen in the autoradiogram (Fig. 6B, lane 1). In the antisense-ODN-treated kidneys, the first-strand cDNA, synthesized by using IR-III primer, had incorporated radioactivity = 1.27 × 107 cpm/μg of RNA. The corresponding autoradiogram revealed faint bands of 3- to 4-kb size besides the smear of radioactivity (Fig. 6B, arrows in lane 3), suggesting that some of the IR transcripts may be intact. The first-strand cDNA, generated by using antisense IR-II primer, had markedly reduced radio-incorporation (0.11 × 107 cpm/μg of RNA) compared with the cDNA synthesized with IR-III as the primer, and the radioactivity was barely discernible in the autoradiogram (Fig. 6B, lane 2 vs. lane 3).
Next, the status of the β subunit was analyzed by PCR in these three first-strand cDNAs containing equal amounts of [32P]dCTP-associated incorporated radioactivity. A PCR product of ≈0.45 kb was observed by agarose gel electrophoresis when control cDNA from untreated explants and the IR-I and -II primer set were employed (Fig. 6C, lane 1). The use of the IR-I and -III primer set resulted in an ≈0.4-kb PCR product (Fig. 6C, lane 2). These products had sequences identical to those reported for mouse 3T3-L1 adipocyte insulin proreceptor (24). When the IR-I and -III primer set and cDNA of antisense-ODN-treated explants, primed with IR-III was used, a PCR product of ≈0.4 kb was detected, and the electrophoretic band had intensity similar to that of the control (Fig. 6C, lane 4 vs. lane 2). A PCR product of ≈0.45 kb was detected by gel electrophoresis when the IR-I and -II primer set and cDNA of antisense-ODN-treated explants, primed with IR-II, were used (Fig. 6C, lane 3). Interestingly, the band intensity was notably less (≈5-fold) as compared with the control (Fig. 6C, lane 3 vs. lane 1). The selective reduction in the synthesis of IR-II-primed specific cDNA and amplification of ≈0.45-kb PCR product suggest that the antisense ODN induced a translational arrest at the site where it bound to the β subunit of the IR gene, and the translation of the α subunit, however, was unaffected, as reflected in the immunoprecipitation experiments (Fig. 5C, lane 3).
Status of ECM Proteins.
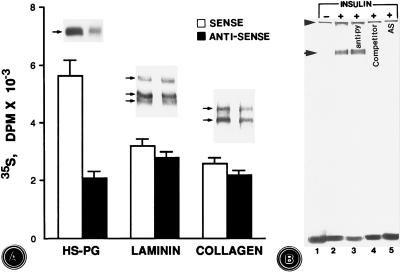
The major matrix morphogenetic proteins which were investigated in the antisense-ODN-treated explants included proteoglycans, laminin, and type IV collagen. All three regulators of morphogenesis had decreased [35S]methionine-associated incorporated radioactivity to various degrees (Fig. 7A, bar graphs). The decrease in the incorporated radioactivities, especially in the proteoglycan fraction, could be seen in the autoradiograms as well (Fig. 7A).
Figure 7.
(A) Profiles of immunoprecipitated radioactivity associated with heparan sulfate-proteoglycan (HS-PG), laminin, and type IV collagen in the extracts of metanephric explants treated with sense and antisense ODNs. A notable decrease in the radioactivity associated with proteoglycan fraction was observed with antisense-ODN treatment, which is also reflected in the corresponding SDS/PAGE autoradiograms. A mild degree of reduction in the incorporated radioactivity is seen in the laminin and type IV collagen as well. Data in bar graphs are expressed as dpm/μg of protein. (B) Autoradiogram showing the effect of insulin on the induction of c-fos promoter region binding protein. With the exposure of metanephric explants to insulin, a nuclear protein that binds to ATP[γ-35S]-labeled dsODN is induced (arrow, lane 2 vs. lane 1). No shift in the band of radioactivity is seen when a mixture of nuclear extract and the radiolabeled dsODN was incubated with anti-phosphotyrosine antibody (lane 3). The unlabeled dsODN competitively inhibited the binding of radiolabeled dsODN with the nuclear protein, since no band of radioactivity is seen in lane 4. No band of radioactivity is seen in the nuclear extracts of explants treated with antisense ODN (AS) in the presence of insulin (lane 5). Arrowhead indicates the point of application of the sample.
DNA–Protein Binding Analysis.
Ligand–receptor (insulin–IR) interaction is followed by phosphorylation of various transcription factors (proteins) which bind to the promoter regions of various IEGs—e.g., c-fos (5, 29). With the exposure of metanephric explants to insulin, a c-fos promoter-binding protein was induced, as indicated by a high molecular band in Fig. 7B, lane 2 vs. lane 1. The phosphorylation of the c-fos binding protein was not confined to the tyrosine residues, since no shift in the band was observed when nuclear extracts of the explants and the radiolabeled c-fos dsODN were incubated with anti-phosphotyrosine antibody (Fig. 7B, lane 3). This DNA–protein interaction seemed to be specific, since no autoradiographic band was observed when a 10-fold excess of the competitor—i.e., unlabeled c-fos dsODN—was included in the reaction mixture (Fig. 7B, lane 4). Also, no autoradiographic band was seen in nuclear extracts of metanephric explants concomitantly exposed to insulin and IR antisense ODN (Fig. 7B, lane 5), suggesting that the disruption of ligand–receptor interaction led to the failure in the phosphorylation of the c-fos binding protein or transcription factor.
DISCUSSION
Over the last decade, a substantial amount of literature data has accumulated to indicate the relevance of the insulin family of peptides and their receptors in very early embryonic development—i.e., in preimplantation embryos (30). However, relatively few attempts have been made to elucidate the spatiotemporal expression of the insulin family of receptors in various organ systems, where they could serve as targets for their respective ligands to modulate cellular proliferation following differentiation of pluripotent cells, a process characteristic of embryonic growth. This communication describes the expression of insulin and IR and their role in metanephric development.
The fact that IR exhibited a spatiotemporal expression (Figs. 1 and 2A) in the embryonic kidney suggests that it has a potential role in nephrogenesis. Furthermore, the presence of IR on the ureteric bud branches and nephron precursors is reminiscent of the expression of the IGF-I receptor (IGF-IR), which plays a vital role in renal development (31). Like IR, the IGF-IR is a transmembrane peptide with an intracellular tyrosine kinase catalytic domain confined to the β subunit. It receives inductive signals from its ligand, IGF-I, apparently expressed in mesenchyme in a paracrine manner to mediate epithelial–mesenchymal interactions so as to sustain early metanephrogenesis (32). Conceivably, similar ligand–receptor interactions are operative between the insulin and its receptor to modulate metanephric growth. Although the exact topological expression of the proinsulin-I and -II genes in the embryonic kidney remains to be determined, the fact that they are expressed in the kidney (Fig. 2B) make it quite conceivable that insulin may mediate its effects in a paracrine manner, besides its endocrine mode of action. These effects include hypertrophy/hyperplasia of the embryonic kidney and increased [3H]thymidine incorporation (Fig. 3B). Such mitogenic effects have been observed in various cell culture systems and preimplantation embryos (8, 14), and the effects in individual organ systems either have not been investigated or insulin was found to be inhibitory for cell growth (33, 34). The latter effect may be due to the interference in the IGF-I–IGF-IR interaction by the insulin at its higher concentrations (1). Furthermore, the data of chicken embryo culture studies suggest that insulin has a stimulatory effect at low concentrations while being toxic or inhibitory at high concentrations (1). Such a concept is partly supported by our findings as well, since the stimulatory effect in metanephric growth was restricted to a concentration of 5 × 10−8 M insulin. These findings also indicate that IR, expressed in the metanephros, is very sensitive to low concentrations of insulin, as observed in other systems (14). This would imply that insulin may play a role in vivo as well, since at day 13 the embryonic kidney is not vascularized and the local tissue concentration of insulin, as reflected by PCR analyses, may be sufficient to sustain metanephric growth in the initial stages of development. It is interesting to note here that the stage of embryonic development has been reported to be a critical factor, among certain others, which dictates the relative efficacy of insulin in terms of its mitogenic potential (13, 35).
How insulin mediates its mitogenic effects is debatable. Some studies suggest that insulin utilizes its own receptor (36), while others have raised the possibility that the growth-promoting effects are mediated by the IGF-IR either because of a high degree of sequence homology between the receptors (6) or by a IGF-IR/IR hybrid receptor (37). The data of this investigation support the first possibility, since disruption of IR by the antisense ODN in the presence of insulin resulted in dysmorphogenesis of the embryonic kidney (Fig. 4C). The specificity of the insulin effect, in terms of utilization of its own receptor, was further reflected by immunoprecipitation studies in which a translational blockade of IR was observed (Fig. 5C). Unexpectedly, the translational blockade was found to be mainly confined to the β subunit of the receptor. In our previous antisense-ODN studies on IGF-IR, translational blockade of both the α and β subunits was noted, which may be related to the fact the antisense ODN was targeted to a site in the α subunit. Thus, RT-PCR analyses were performed upstream of the antisense-ODN binding regions to establish that the mRNA cleavage occurred at that particular site after RNase-H activation in the antisense-treated metanephric explants. The fact that amplification of cDNA segments upstream of the antisense-ODN binding sites was unaffected (Fig. 6C) could explain why the translational blockade was confined to the β subunit, while the α subunit of IR protein was unaffected. Whether such a differential translational blockade can be achieved in other transmembrane receptor proteins with α and β subunits, which are involved in paracrine interactions, remains to be investigated.
In concert with such growth factor–receptor interactions, the epithelial–mesenchymal interactions also play a vital role in the organogenesis of the mammalian metanephros (21). The epithelial–mesenchymal interactions are dependent upon proper intercalation of the ureteric bud branches into the loose mesenchyme, and expression of ECM macromolecules on their budding tips (21). The synchronous nature of these paracrine interactions is well exemplified by the findings of this study, where dysmorphogenesis of the ureteric bud branches (Fig. 4C) was accompanied with decreased de novo synthesis of ECM proteins, especially that of the proteoglycans (Fig. 7A). The latter are well known regulators of organogenesis in embryonic development (38), and their selective susceptibility to antisense ODN may be related to their high turnover during remodeling of the ECM (21).
Alternatively, since insulin is known to activate transcription factor AP2, which then binds to the promoter region of the heparan sulfate proteoglycan (HS-PG) (39), the antisense-ODN-induced perturbation in the insulin–IR interaction may have caused a failure in the activation of AP2 leading to decreased de novo synthesis of HS-PG.
In recent years, transcription factors and various protooncogenes have also been implicated in the organogenesis of the mammalian metanephros (21). Among the various protooncogenes, extensive studies have been performed on the molecular organization of IEGs—i.e., c-fos, c-jun, and Egr-1, which are extremely pertinent to cell growth (5). Interestingly, some of the IEGs—e.g., c-fos and Egr-1, display coregulatory induction kinetics under the influence of growth-promoting stimuli (40). Since insulin, a mitogen, has been reported to induce c-fos mRNA expression in neuroblastoma cells (36), we investigated whether the perturbation of ligand–receptor interactions leads to an interference in the activation and binding of transcription factor(s) to the promoter region of c-fos and ceases its induction with consequential retardation in the metanephric growth. The fact that there was failure in the activation of the c-fos DNA-binding protein in the renal explants treated with antisense ODN, as revealed by gel-shift assays (Fig. 7B), would suggest that the induction of c-fos mRNA may be critical for the metanephric growth. Intriguingly, the c-fos promoter region binding protein, which is activated upon insulin stimulation, is not phosphorylated at the tyrosine residues (Fig. 7B). Its biochemical characterization would be an interesting area of future investigations because it seems to have crucial relevance in mammalian organogenesis, which are modulated by insulin–insulin receptor interactions during early embryonic life.
Acknowledgments
This work was supported by National Institutes of Health Grant DK28492.
ABBREVIATIONS
- IGF
insulin-like growth factor
- IGF-IR
IGF-I receptor
- IEG
immediate-early gene
- IR
insulin receptor
- ECM
extracellular matrix
- RT
reverse transcriptase
- ATP[γ-35S]
adenosine 5′-[γ-[35S]thio]triphosphate
- ODN
oligodeoxynucleotide
- dsODN
double-stranded ODN
Footnotes
One day-13 metanephric explant yields ≈0.5 μg of RNA, and about 3,000 explants were used in all the experiments of this investigation.
References
- 1.de Pablo F, Scott L A, Roth J. Endocr Rev. 1990;11:558–577. doi: 10.1210/edrv-11-4-558. [DOI] [PubMed] [Google Scholar]
- 2.Drummond I A, Madden S L, Nutter P R, Bell G I, Rauscher F J, Sukhatme V P. Science. 1992;257:674–678. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- 3.Cohick W S, Clemmons D R. Annu Rev Physiol. 1993;55:131–153. doi: 10.1146/annurev.ph.55.030193.001023. [DOI] [PubMed] [Google Scholar]
- 4.van der Geer P, Hunter T, Lindberg R A. Ann Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- 5.Gashler A G, Sukhatme V P. Prog Nucleic Acid Res Mol Biol. 1995;50:191–224. doi: 10.1016/s0079-6603(08)60815-6. [DOI] [PubMed] [Google Scholar]
- 6.LeRoith D, Sampson P C, Roberts C T. Horm Res. 1994;41:74–79. doi: 10.1159/000183964. [DOI] [PubMed] [Google Scholar]
- 7.Serrano J, Bevins C L, Young S W, de Pablo F. Dev Biol. 1989;132:410–418. doi: 10.1016/0012-1606(89)90237-6. [DOI] [PubMed] [Google Scholar]
- 8.Heyner S, Rao L V, Jarett L, Smith R M. Dev Biol. 1989;134:48–58. doi: 10.1016/0012-1606(89)90077-8. [DOI] [PubMed] [Google Scholar]
- 9.Antin P B, Yatskievych T, Dominguez L, Chieffi P. J Cell Physiol. 1996;168:42–50. doi: 10.1002/(SICI)1097-4652(199607)168:1<42::AID-JCP6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Thiet M-P, Osathanondh R, Yeh P. Am J Obstet Gynecol. 1994;170:152–156. doi: 10.1016/s0002-9378(94)70401-5. [DOI] [PubMed] [Google Scholar]
- 11.Muglia L, Locker J. Proc Natl Acad Sci USA. 1984;81:3635–3639. doi: 10.1073/pnas.81.12.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giddings S J, Carnaghi L. J Biol Chem. 1989;264:9462–9469. [PubMed] [Google Scholar]
- 13.Spaventi R, Antica M, Pavelic K. Development (Cambridge, UK) 1989;108:491–495. doi: 10.1242/dev.108.3.491. [DOI] [PubMed] [Google Scholar]
- 14.Harvey M B, Kaye P L. Development (Cambridge, UK) 1990;110:963–967. doi: 10.1242/dev.110.3.963. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez C H, Carranza A L, Alarcon C, de La Rosa E J, de Pablo F. Proc Natl Acad Sci USA. 1995;92:9834–9838. doi: 10.1073/pnas.92.21.9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Jack J, Garoofalo R S. Endocrinology. 1996;137:846–856. doi: 10.1210/endo.137.3.8603594. [DOI] [PubMed] [Google Scholar]
- 17.Joshi R L, Lamothe B, Cordonnier N, Mesbah K, Monthioux E, Jami J, Bucchini D. EMBO J. 1996;15:1542–1547. [PMC free article] [PubMed] [Google Scholar]
- 18.Venkatachalam M A, Kriz W. In: Pathology of Kidney. Heptinstall R H, editor. Boston: Little Brown; 1992. pp. 1–93. [Google Scholar]
- 19.Ekblom P. In: Molecular and Cellular Aspects of Basement Membranes. Rohrbach D H, Rupert T, editors. New York: Academic; 1993. pp. 359–383. [Google Scholar]
- 20.Clapp W L, Abrahamson D R. In: Renal Pathology. Craig C, Brenner B M, editors. Philadelphia: Lippincott; 1994. pp. 3–59. [Google Scholar]
- 21.Kanwar, Y., Carone, F., Wada, J., Ota, K. & Wallner, E. (1997) Kidney Int. 51, in press. [DOI] [PubMed]
- 22.Price R G, Hudson B G. In: Renal Basement Membranes in Health and Disease. Price R G, Hudson B G, editors. New York: Academic; 1987. pp. 3–9. [Google Scholar]
- 23.Liu Z Z, Wada J, Kumar A, Carone F A, Takahashi M, Kanwar Y S. Dev Biol. 1996;178:133–148. doi: 10.1006/dbio.1996.0204. [DOI] [PubMed] [Google Scholar]
- 24.Flores-Riveros J R, Sibley E, Kastelic T, Lane M D. J Biol Chem. 1989;264:21557–21572. [PubMed] [Google Scholar]
- 25.Chirgwin J M, Pryzbyla A E, MacDonald R J, Rutter W J. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 26.Wentworth B M, Schaefer I M, Villa-Komaroff L V, Chirgwin J M. J Mol Evol. 1986;23:305–312. doi: 10.1007/BF02100639. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein J, Chen F, Roszka J. Lab Invest. 1981;45:183–190. [PubMed] [Google Scholar]
- 28.Dignam J D, Lebovitz R M, Roeder R G. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruff-Jamison S, Chen K, Cohen S. Science. 1993;261:1733–1736. doi: 10.1126/science.8378774. [DOI] [PubMed] [Google Scholar]
- 30.Heyner S, Farber M, Rosenblum I Y. Curr Top Dev Biol. 1993;24:137–159. doi: 10.1016/s0070-2153(08)60086-1. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z Z, Wada J, Alvares K, Kumar A, Wallner E I, Kanwar Y S. Kidney Int. 1993;44:1242–1250. doi: 10.1038/ki.1993.375. [DOI] [PubMed] [Google Scholar]
- 32.Wada J, Liu Z Z, Alvares K, Kumar A, Wallner E I, Makino H, Kanwar Y S. Proc Natl Acad Sci USA. 1993;90:10360–10364. doi: 10.1073/pnas.90.21.10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weller A, Sorokin L, Illgen E-V, Ekblom P. Dev Biol. 1991;144:248–261. doi: 10.1016/0012-1606(91)90419-4. [DOI] [PubMed] [Google Scholar]
- 34.Chailler P, Briere N. Cell Biol Int Rep. 1991;15:955–962. doi: 10.1016/0309-1651(91)90145-9. [DOI] [PubMed] [Google Scholar]
- 35.Ridpath J F, Huiatt T W, Trenkle A H, Robsom R M, Bechtel P J. Differentiation. 1984;26:121–126. doi: 10.1111/j.1432-0436.1984.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 36.Mattsson M E, Hammerling U, Mohall E, Hall K, Pahlman S. Growth Factors. 1990;2:251–265. doi: 10.3109/08977199009167020. [DOI] [PubMed] [Google Scholar]
- 37.Soos M A, Siddle K. Biochem J. 1989;263:553–563. doi: 10.1042/bj2630553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toole B P. In: Cell Biology of Extracellular Matrix. Hay E D, editor. New York: Plenum; 1991. pp. 305–342. [Google Scholar]
- 39.Kanwar Y S, Liu Z Z, Kumar A, Usman I M, Wada J, Wallner E I. J Clin Invest. 1996;98:2478–2488. doi: 10.1172/JCI119066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sukhatme V P, Cao X, Chang L C, Tsai-Morris C H, Stamenkovich D, Ferreira P C P, Cohen D R, Edwards S A, Shows T B, Curran T, LeBeau M M, Adamson E D. Cell. 1988;53:37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]