Abstract
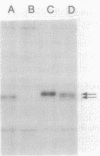
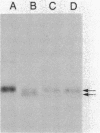
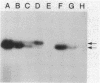
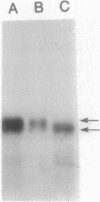
We have fused the structural gene (hsa) for human serum albumin (HSA) to the expression elements and signal sequence coding region of each of two genes from Bacillus amyloliquefaciens P, an alpha-amylase gene (amyBamP) and a neutral protease gene (nprBamP). Bacillus subtilis strains harboring either of these gene fusions synthesized a protein with the antigenic characteristics and size (68 kilodaltons) of HSA. Results from pulse-labeling studies indicated that the bacterially produced HSA was secreted from cells which had been converted to protoplasts. Results from similar studies with intact cells suggested that the signal sequence was removed from the hybrid protein, providing further evidence that B. subtilis can translocate this foreign protein across the cell membrane. Signal sequence removal was efficient when the level of HSA synthesis was low. However, in strains which synthesized HSA at a high level, signal sequence removal was less efficient.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anba J., Lazdunski C., Pages J. M. Lack of effect of leader peptidase overproduction on the processing in vivo of exported proteins in Escherichia coli. J Gen Microbiol. 1986 Mar;132(3):689–696. doi: 10.1099/00221287-132-3-689. [DOI] [PubMed] [Google Scholar]
- Bakker E. P., Randall L. L. The requirement for energy during export of beta-lactamase in Escherichia coli is fulfilled by the total protonmotive force. EMBO J. 1984 Apr;3(4):895–900. doi: 10.1002/j.1460-2075.1984.tb01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Daniels C. J., Bole D. G., Quay S. C., Oxender D. L. Role for membrane potential in the secretion of protein into the periplasm of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5396–5400. doi: 10.1073/pnas.78.9.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Goodman J. M., Wickner W. T. Procoat, the precursor of M13 coat protein, requires an electrochemical potential for membrane insertion. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4669–4673. doi: 10.1073/pnas.77.8.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Law S. W., Dennison O. E. Nucleotide sequence and the encoded amino acids of human serum albumin mRNA. Proc Natl Acad Sci U S A. 1982 Jan;79(1):71–75. doi: 10.1073/pnas.79.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emr S. D., Hedgpeth J., Clément J. M., Silhavy T. J., Hofnung M. Sequence analysis of mutations that prevent export of lambda receptor, an Escherichia coli outer membrane protein. Nature. 1980 May 8;285(5760):82–85. doi: 10.1038/285082a0. [DOI] [PubMed] [Google Scholar]
- Fisher S. H., Sonenshein A. L. Bacillus subtilis glutamine synthetase mutants pleiotropically altered in glucose catabolite repression. J Bacteriol. 1984 Feb;157(2):612–621. doi: 10.1128/jb.157.2.612-621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T., Shivakumar A. G., Dubnau D. Characterization of chimeric plasmid cloning vehicles in Bacillus subtilis. J Bacteriol. 1980 Jan;141(1):246–253. doi: 10.1128/jb.141.1.246-253.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bassford P. J., Jr, Beckwith J. Protein localization in E. coli: is there a common step in the secretion of periplasmic and outer-membrane proteins? Cell. 1981 Jun;24(3):707–717. doi: 10.1016/0092-8674(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Judah J. D., Quinn P. S. Calcium ion-dependent vesicle fusion in the conversion of proalbumin to albumin. Nature. 1978 Jan 26;271(5643):384–385. doi: 10.1038/271384a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Adelman J., Bock S. C., Franke A. E., Houck C. M., Najarian R. C., Seeburg P. H., Wion K. L. The sequence of human serum albumin cDNA and its expression in E. coli. Nucleic Acids Res. 1981 Nov 25;9(22):6103–6114. doi: 10.1093/nar/9.22.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Duvall E. J., Keggins K. M. Bacillus pumilus plasmid pPL10: properties and insertion into Bacillus subtilis 168 by transformation. J Bacteriol. 1976 Aug;127(2):817–828. doi: 10.1128/jb.127.2.817-828.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Hunt J. F., Beckwith J. Effects of signal sequence mutations on the kinetics of alkaline phosphatase export to the periplasm in Escherichia coli. J Bacteriol. 1986 Jul;167(1):160–167. doi: 10.1128/jb.167.1.160-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkley E. G., Jr Purification and characterization of pro-TraTp, the signal sequence-containing precursor of a secreted protein encoded by the F sex factor. J Bacteriol. 1984 May;158(2):464–473. doi: 10.1128/jb.158.2.464-473.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murén E. M., Randall L. L. Export of alpha-amylase by Bacillus amyloliquefaciens requires proton motive force. J Bacteriol. 1985 Nov;164(2):712–716. doi: 10.1128/jb.164.2.712-716.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Holmgren E., Josephson S., Gatenbeck S., Philipson L., Uhlen M. Efficient secretion and purification of human insulin-like growth factor I with a gene fusion vector in Staphylococci. Nucleic Acids Res. 1985 Feb 25;13(4):1151–1162. doi: 10.1093/nar/13.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell. 1981 Sep;25(3):765–772. doi: 10.1016/0092-8674(81)90184-7. [DOI] [PubMed] [Google Scholar]
- Palva I., Lehtovaara P., Käriäinen L., Sibakov M., Cantell K., Schein C. H., Kashiwagi K., Weissmann C. Secretion of interferon by Bacillus subtilis. Gene. 1983 May-Jun;22(2-3):229–235. doi: 10.1016/0378-1119(83)90107-5. [DOI] [PubMed] [Google Scholar]
- Palva I., Pettersson R. F., Kalkkinen N., Lehtovaara P., Sarvas M., Söderlund H., Takkinen K., Käriäinen L. Nucleotide sequence of the promoter and NH2-terminal signal peptide region of the alpha-amylase gene from Bacillus amyloliquefaciens. Gene. 1981 Oct;15(1):43–51. doi: 10.1016/0378-1119(81)90103-7. [DOI] [PubMed] [Google Scholar]
- Palva I., Sarvas M., Lehtovaara P., Sibakov M., Käriäinen L. Secretion of Escherichia coli beta-lactamase from Bacillus subtilis by the aid of alpha-amylase signal sequence. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5582–5586. doi: 10.1073/pnas.79.18.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph C. F., Schmidt B. J., Saunders C. W. Transformation of Bacillus subtilis by single-stranded plasmid DNA. J Bacteriol. 1986 Mar;165(3):1015–1018. doi: 10.1128/jb.165.3.1015-1018.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C. W., Schmidt B. J., Mirot M. S., Thompson L. D., Guyer M. S. Use of chromosomal integration in the establishment and expression of blaZ, a Staphylococcus aureus beta-lactamase gene, in Bacillus subtilis. J Bacteriol. 1984 Mar;157(3):718–726. doi: 10.1128/jb.157.3.718-726.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B. J., Strasser J., Saunders C. W. A Bacillus subtilis plasmid that can be packaged as single-stranded DNA in Escherichia coli: use for oligodeoxynucleotide-directed mutagenesis. Gene. 1986;41(2-3):331–335. doi: 10.1016/0378-1119(86)90116-2. [DOI] [PubMed] [Google Scholar]
- Shiroza T., Nakazawa K., Tashiro N., Yamane K., Yanagi K., Yamasaki M., Tamura G., Saito H., Kawade Y., Taniguchi T. Synthesis and secretion of biologically active mouse interferon-beta using a Bacillus subtilis alpha-amylase secretion vector. Gene. 1985;34(1):1–8. doi: 10.1016/0378-1119(85)90288-4. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Ofek I., Beachey E. H. Binding of streptococcal lipoteichoic acid to the fatty acid binding sites on serum albumin. J Biol Chem. 1980 Jul 10;255(13):6092–6097. [PubMed] [Google Scholar]
- Smith R. A., Duncan M. J., Moir D. T. Heterologous protein secretion from yeast. Science. 1985 Sep 20;229(4719):1219–1224. doi: 10.1126/science.3939723. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Ulmanen I., Lundström K., Lehtovaara P., Sarvas M., Ruohonen M., Palva I. Transcription and translation of foreign genes in Bacillus subtilis by the aid of a secretion vector. J Bacteriol. 1985 Apr;162(1):176–182. doi: 10.1128/jb.162.1.176-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasantha N., Freese E. Enzyme changes during Bacillus subtilis sporulation caused by deprivation of guanine nucleotides. J Bacteriol. 1980 Dec;144(3):1119–1125. doi: 10.1128/jb.144.3.1119-1125.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasantha N., Thompson L. D., Rhodes C., Banner C., Nagle J., Filpula D. Genes for alkaline protease and neutral protease from Bacillus amyloliquefaciens contain a large open reading frame between the regions coding for signal sequence and mature protein. J Bacteriol. 1984 Sep;159(3):811–819. doi: 10.1128/jb.159.3.811-819.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasantha N., Thompson L. D. Secretion of a heterologous protein from Bacillus subtilis with the aid of protease signal sequences. J Bacteriol. 1986 Mar;165(3):837–842. doi: 10.1128/jb.165.3.837-842.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Redman C. In vitro synthesis of rat pre-proalbumin. Biochem Biophys Res Commun. 1976 May 23;76(2):469–476. doi: 10.1016/0006-291x(77)90748-3. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]