Abstract
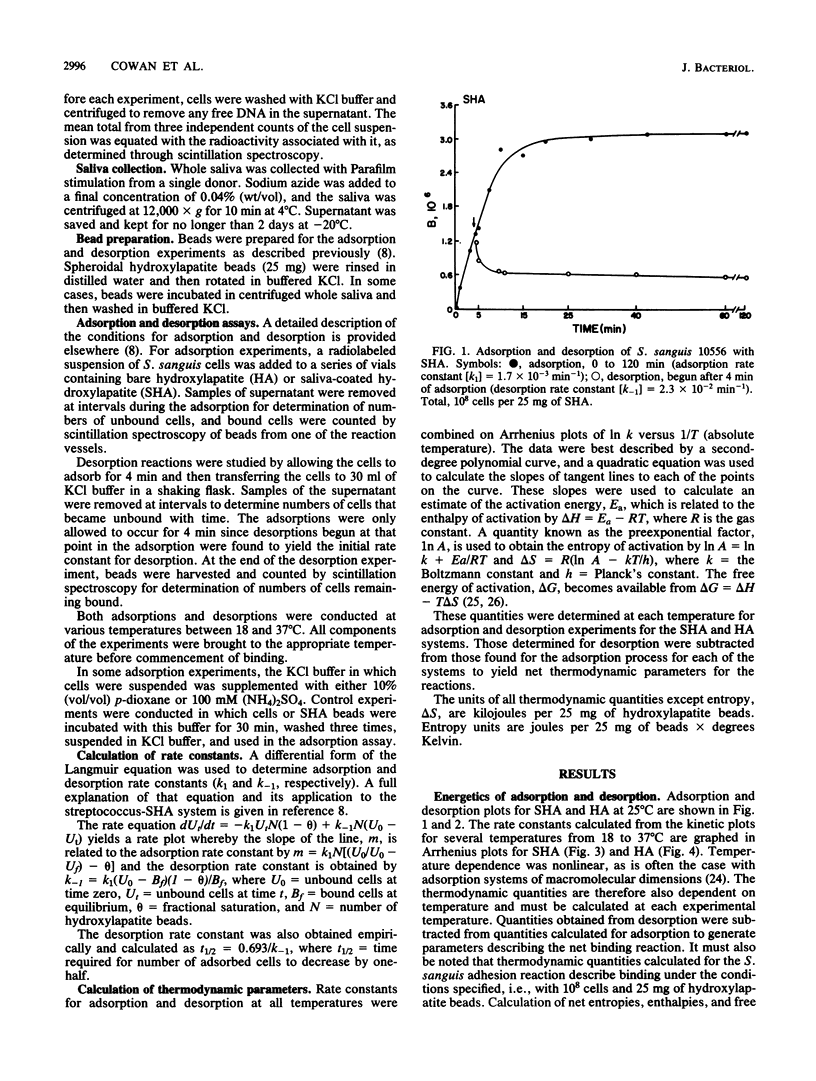
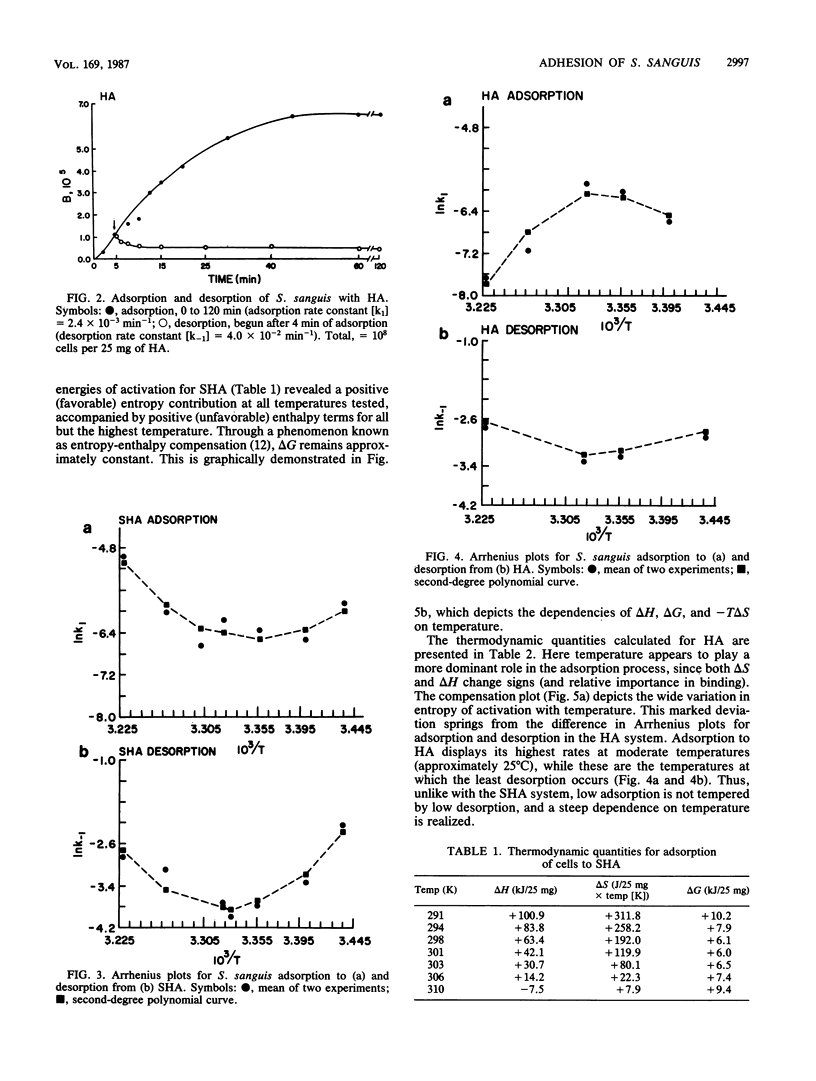
The initial adhesion of Streptococcus sanguis 10556 to artificial salivary pellicle and to bare hydroxylapatite was studied at several temperatures between 18 and 37 degrees C. When the natural logarithms of rate constants for adsorption and desorption were plotted against reciprocal temperatures in Arrhenius plots, curved lines were obtained, indicating that the thermodynamic quantities of enthalpy and entropy of activation were temperature dependent. For the bare hydroxylapatite system, the heat capacity (delta Cp = dH/dT) was large and negative. delta Cp was also negative for adhesion to saliva-coated hydroxylapatite, although its value was lower. Negative heat capacities, when coupled with favorable entropy, are often indicative of either electrostatic or hydrophobic interactions. When electrolyte (100 mM ammonium sulfate) was added to the cell-hydroxylapatite bead mixture, the rate and extent of adhesion were decreased. Addition of nonpolar p-dioxane (10% [vol/vol], final concentration) to the mixture enhanced binding. This suggests that electrostatic linkages participate in the primary adhesion of streptococci to both substrata. The strongly positive entropy values and the lesser temperature dependence of the saliva-coated hydroxylapatite system suggest that another entropy-driven process is imposed on the electrostatic linkages. This supports a role for hydrophobicity, suggesting that a combination of electrostatic and hydrophobic forces mediate the initial adhesion of S. sanguis to the salivary pellicle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absolom D. R., Lamberti F. V., Policova Z., Zingg W., van Oss C. J., Neumann A. W. Surface thermodynamics of bacterial adhesion. Appl Environ Microbiol. 1983 Jul;46(1):90–97. doi: 10.1128/aem.46.1.90-97.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absolom D. R., Neumann A. W., Zingg W., van Oss C. J. Thermodynamic studies of cellular adhesion. Trans Am Soc Artif Intern Organs. 1979;25:152–158. doi: 10.1097/00002480-197902500-00029. [DOI] [PubMed] [Google Scholar]
- Busscher H. J., Uyen M. H., van Pelt A. W., Weerkamp A. H., Arends J. Kinetics of adhesion of the oral bacterium Streptococcus sanguis CH3 to polymers with different surface free energies. Appl Environ Microbiol. 1986 May;51(5):910–914. doi: 10.1128/aem.51.5.910-914.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan M. M., Taylor K. G., Doyle R. J. Kinetic analysis of Streptococcus sanguis adhesion to artificial pellicle. J Dent Res. 1986 Oct;65(10):1278–1283. doi: 10.1177/00220345860650101501. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Comparative hydrophobicities of oral bacteria and their adherence to salivary pellicles. Infect Immun. 1983 Sep;41(3):1190–1196. doi: 10.1128/iai.41.3.1190-1196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I., Moreno E. C. Association of neuraminidase-sensitive receptors and putative hydrophobic interactions with high-affinity binding sites for Streptococcus sanguis C5 in salivary pellicles. Infect Immun. 1983 Dec;42(3):1006–1012. doi: 10.1128/iai.42.3.1006-1012.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron J. N., Kranz D. M., Jameson D. M., Voss E. W., Jr Thermodynamic properties of ligand binding by monoclonal anti-fluorescyl antibodies. Biochemistry. 1986 Aug 12;25(16):4602–4609. doi: 10.1021/bi00364a022. [DOI] [PubMed] [Google Scholar]
- Kilpatrick G. J., el Tayar N., Van de Waterbeemd H., Jenner P., Testa B., Marsden C. D. The thermodynamics of agonist and antagonist binding to dopamine D-2 receptors. Mol Pharmacol. 1986 Sep;30(3):226–234. [PubMed] [Google Scholar]
- Lavialle F., Rogard M., Alfsen A. Calorimetric determination of the enthalpy and heat capacity changes for the association of haptoglobin with hemoglobin. I. Demonstration of two interacting systems. Biochemistry. 1974 May 7;13(10):2231–2234. doi: 10.1021/bi00707a032. [DOI] [PubMed] [Google Scholar]
- Moreno E. C., Kresak M., Hay D. I. Adsorption thermodynamics of acidic proline-rich human salivary proteins onto calcium apatites. J Biol Chem. 1982 Mar 25;257(6):2981–2989. [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G. Hydrophobic interactions and the adherence of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Nov;38(2):637–644. doi: 10.1128/iai.38.2.637-644.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niekamp C. W., Sturtevant J. M., Velick S. F. Energetics of the cooperative and noncooperative binding of nicotinamide adenine dinucleotide to yeast glyceraldehyde-3-phosphate dehydrogenase at pH 6.5 and pH 8.5. Equilibrium and calorimetric analysis over a range of temperature. Biochemistry. 1977 Feb 8;16(3):436–445. doi: 10.1021/bi00622a015. [DOI] [PubMed] [Google Scholar]
- Ruckenstein E., Srinivasan R. Comments on cell adhesion to biomaterial surfaces: the origin of saturation in platelet deposition--is it kinetic or thermodynamic? J Biomed Mater Res. 1982 Mar;16(2):169–172. doi: 10.1002/jbm.820160209. [DOI] [PubMed] [Google Scholar]
- Sturtevant J. M. Heat capacity and entropy changes in processes involving proteins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2236–2240. doi: 10.1073/pnas.74.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy M. J., Krishna Sastry M. V., Khan M. I., Surolia A. Thermodynamic and kinetic studies on saccharide binding to soya-bean agglutinin. Biochem J. 1986 Mar 15;234(3):515–522. doi: 10.1042/bj2340515. [DOI] [PMC free article] [PubMed] [Google Scholar]



