Abstract
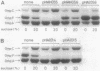
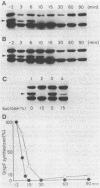
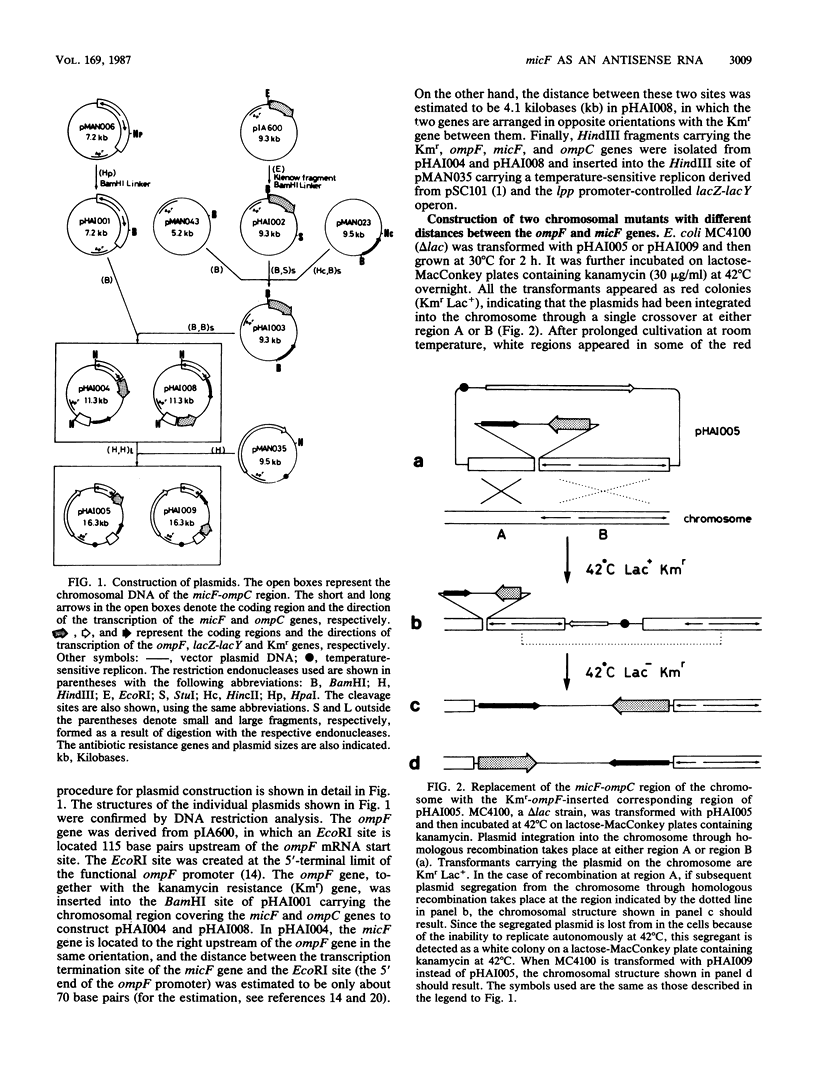
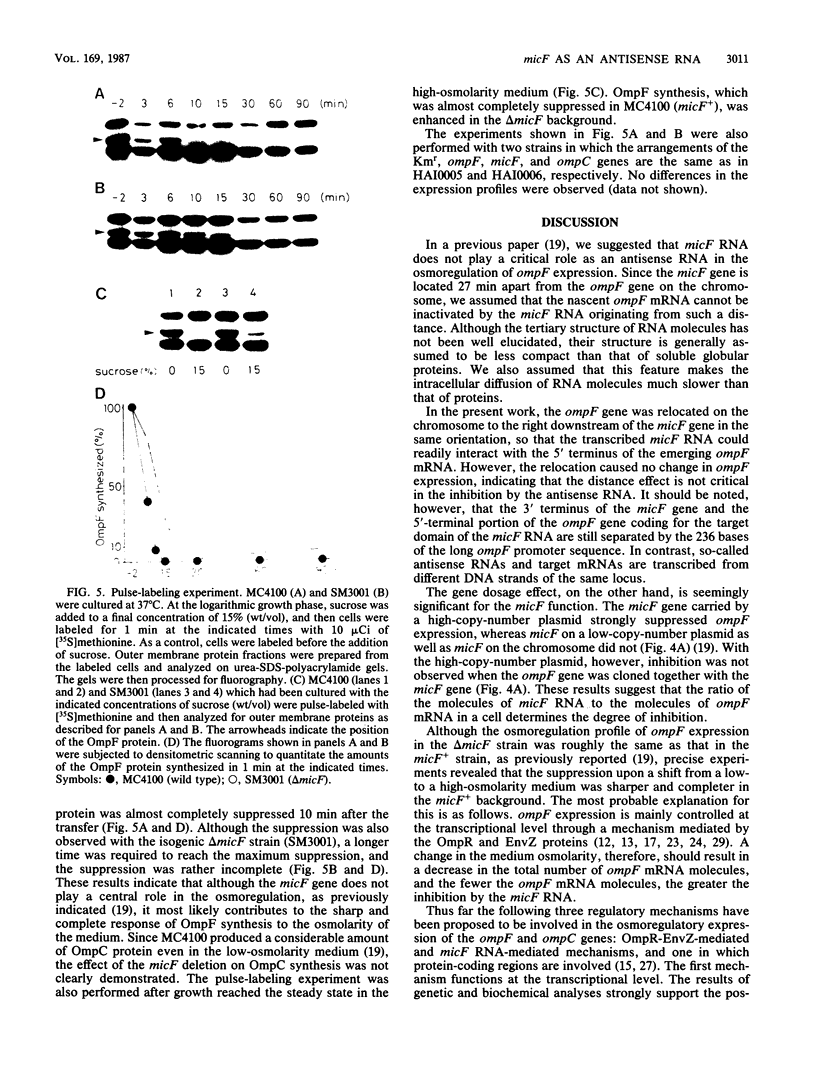
To analyze the function of micF as an antisera RNA in the osmoregulatory expression of the ompF gene in Escherichia coli, we performed two experiments. In the first experiment, two strains were constructed in which the transcription initiation site of the ompF gene and the transcription termination site of the micF gene were separated by 186 and 4,100 base pairs, respectively, on the chromosome. These two strains showed almost the same profile of ompF expression as the wild-type strain in which the two genes are separated by 10(6) base pairs. When a high-copy-number plasmid carrying the micF gene was introduced into these strains, ompF expression was completely repressed, whereas no repression was observed with a low-copy-number plasmid carrying the micF gene. These results indicate that the distance between the two genes on the chromosome is not critical for the function of micF. In the second experiment, expression of the ompF gene was examined by pulse-labeling in both the micF+ and the micF deletion strains. Upon a shift from a low- to a high-osmolarity medium, suppression of OmpF protein synthesis occurred more quickly and more extensively in the micF+ strain than in the micF deletion strain. The steady-state synthesis of the OmpF protein was also completely suppressed in the micF+ strain in the high-osmolarity medium, whereas the suppression was incomplete in the micF deletion strain. From these results we conclude that (i) the micF gene contributes to the fast and complete response of the OmpF synthesis to the medium osmolarity, and that (ii) the distance between the micF and ompF genes on the chromosomes is not critical for the function of the micF gene. The results suggest, rather, that the ratio of the copy numbers of the two genes is critical for the function of the micF gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alphen W. V., Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol. 1977 Aug;131(2):623–630. doi: 10.1128/jb.131.2.623-630.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong K. A., Acosta R., Ledner E., Machida Y., Pancotto M., McCormick M., Ohtsubo H., Ohtsubo E. A 37 X 10(3) molecular weight plasmid-encoded protein is required for replication and copy number control in the plasmid pSC101 and its temperature-sensitive derivative pHS1. J Mol Biol. 1984 May 25;175(3):331–348. doi: 10.1016/0022-2836(84)90352-8. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cabello F., Timmis K., Cohen S. N. Replication control in a composite plasmid constructed by in vitro linkage of two distinct replicons. Nature. 1976 Jan 29;259(5541):285–290. doi: 10.1038/259285a0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Escherichia coli K-12 tolF mutants: alterations in protein composition of the outer membrane. J Bacteriol. 1977 May;130(2):781–786. doi: 10.1128/jb.130.2.781-786.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dairi T., Inokuchi K., Mizuno T., Mizushima S. Positive control of transcription initiation in Escherichia coli. A base substitution at the Pribnow box renders ompF expression independent of a positive regulator. J Mol Biol. 1985 Jul 5;184(1):1–6. doi: 10.1016/0022-2836(85)90038-5. [DOI] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. TolF locus in Escherichia coli: chromosomal location and relationship to loci cmlB and tolD. J Bacteriol. 1976 Nov;128(2):604–608. doi: 10.1128/jb.128.2.604-608.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett S., Taylor R. K., Silhavy T. J. Isolation and characterization of chain-terminating nonsense mutations in a porin regulator gene, envZ. J Bacteriol. 1983 Oct;156(1):62–69. doi: 10.1128/jb.156.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Inokuchi K., Furukawa H., Nakamura K., Mizushima S. Characterization by deletion mutagenesis in vitro of the promoter region of ompF, a positively regulated gene of Escherichia coli. J Mol Biol. 1984 Sep 25;178(3):653–668. doi: 10.1016/0022-2836(84)90243-2. [DOI] [PubMed] [Google Scholar]
- Inokuchi K., Itoh M., Mizushima S. Domains involved in osmoregulation of the ompF gene in Escherichia coli. J Bacteriol. 1985 Nov;164(2):585–590. doi: 10.1128/jb.164.2.585-590.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaji H., Mizuno T., Mizushima S. Influence of molecular size and osmolarity of sugars and dextrans on the synthesis of outer membrane proteins O-8 and O-9 of Escherichia coli K-12. J Bacteriol. 1979 Dec;140(3):843–847. doi: 10.1128/jb.140.3.843-847.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Inokuchi K., Mizushima S. Promoter exchange between ompF and ompC, genes for osmoregulated major outer membrane proteins of Escherichia coli K-12. J Bacteriol. 1984 Jun;158(3):1041–1047. doi: 10.1128/jb.158.3.1041-1047.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Mizuno T., Mizushima S. Interaction between two regulatory proteins in osmoregulatory expression of ompF and ompC genes in Escherichia coli: a novel ompR mutation suppresses pleiotropic defects caused by an envZ mutation. J Bacteriol. 1986 Dec;168(3):1309–1314. doi: 10.1128/jb.168.3.1309-1314.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Mizushima S. Construction and characterization of a deletion mutant lacking micF, a proposed regulatory gene for OmpF synthesis in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1196–1202. doi: 10.1128/jb.162.3.1196-1202.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Mizushima S. Characterization by deletion and localized mutagenesis in vitro of the promoter region of the Escherichia coli ompC gene and importance of the upstream DNA domain in positive regulation by the OmpR protein. J Bacteriol. 1986 Oct;168(1):86–95. doi: 10.1128/jb.168.1.86-95.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara F., Inokuchi K., Matsuyama S., Mizushima S. Mutation causing reverse osmoregulation of synthesis of OmpF, a major outer membrane protein of Escherichia coli. J Bacteriol. 1984 Aug;159(2):688–692. doi: 10.1128/jb.159.2.688-692.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara F., Matsuyama S., Mizuno T., Mizushima S. Molecular analysis of mutant ompR genes exhibiting different phenotypes as to osmoregulation of the ompF and ompC genes of Escherichia coli. Mol Gen Genet. 1986 Feb;202(2):194–199. doi: 10.1007/BF00331636. [DOI] [PubMed] [Google Scholar]
- Nara F., Mizuno T., Mizushima S. Complementation analysis of the wild-type and mutant ompR genes exhibiting different phenotypes of osmoregulation of the ompF and ompC genes of Escherichia coli. Mol Gen Genet. 1986 Oct;205(1):51–55. [PubMed] [Google Scholar]
- Ozawa Y., Mizuno T., Mizushima S. Roles of the Pribnow box in positive regulation of the ompC and ompF genes in Escherichia coli. J Bacteriol. 1987 Mar;169(3):1331–1334. doi: 10.1128/jb.169.3.1331-1334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan G., Ikenaka K., Inouye M. Uncoupling of osmoregulation of the Escherichia coli K-12 ompF gene from ompB-dependent transcription. J Bacteriol. 1985 Jul;163(1):82–87. doi: 10.1128/jb.163.1.82-87.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Hall M. N., Silhavy T. J. Isolation and characterization of mutations altering expression of the major outer membrane porin proteins using the local anaesthetic procaine. J Mol Biol. 1983 May 25;166(3):273–282. doi: 10.1016/s0022-2836(83)80085-0. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T. Plasmid ColE1 incompatibility determined by interaction of RNA I with primer transcript. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6096–6100. doi: 10.1073/pnas.78.10.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]