Abstract
The difficulty in controlling Plasmodium vivax, the most common cause of human malaria, has been complicated by growing drug resistance. We have established a method to cycle parasite generations in continuous culture using human blood cells. Chesson strain parasites were passaged from owl monkey erythrocytes to human reticulocytes in McCoy’s 5A medium modified with L-glutamine with 25 mM Hepes buffer supplemented with 20% AB+ human serum. Reticulocytes were separated by differential centrifugation in homologous plasma from the peripheral blood of a hemochromatosis patient. Parasites were grown during each 48-hr cycle in a static candle jar environment until the beginning of schizogony, at about 36–40 hr, when reticulocytes were added and cultures transferred to a shaker for 10–12 hr. The addition of a concentration of 10% reticulocytes resulted in stabilizing parasite densities between 0.28 and 0.57 after cycle 3 and increasing the total number of parasites at least 2-fold with each generational cycle. Cultured parasites successfully infected an owl monkey. The morphology of cultured parasites was typical of P. vivax, with highly ameboid trophozoites evident; however, infected erythrocytes were enlarged and distorted on thin film preparations. The species identity of cultivated parasites was confirmed by analysis of the A and C 18S rRNA genes from genomic DNA and expression of only the A gene during erythrocytic asexual growth. The ability to culture P. vivax opens new opportunities to develop vaccines, test drugs, and clone parasites for genome sequencing.
Plasmodium vivax is the most widespread and, except in equatorial Africa, the most prevalent malaria infection of man. While 3 million cases were reported in 1992 (1), it is widely acknowledged that at least ten times as many cases occur annually. It produces a severe, fulminant illness that can relapse months after the original infection as dormant parasites are released from the liver. Successful therapy requires clearance of both the blood and liver stages. Treatment has been complicated by the recent emergence of chloroquine-resistant P. vivax in Southeast Asia (2–4); chloroquine, which is safe and inexpensive, has been the drug of choice for eliminating the blood stages. No vaccine exists and genetic research lags far behind that done on Plasmodium falciparum, the other major human malaria. Much of the current research on P. falciparum is possible because of the discovery, more than 20 years ago, of a method for its continuous in vitro propagation (5). Methods have since been developed for culturing the simian malarias Plasmodium inui (6) and the vivax-like Plasmodium cynomolgi (7), but until now, all attempts to develop an in vitro method for P. vivax have failed.
Crucial to the success of any method for P. vivax cultivation is the availability of sufficient numbers of susceptible red blood cells for the parasite to invade. During the erythrocytic stage of development, or schizogony, the haploid P. vivax parasites undergo repeated, synchronized 48-hr cycles of multiplication. After invading a red blood cell, the merozoite differentiates first to the ring and then to the trophozoite stage before beginning nuclear divisions that produce the schizont, which typically contains 16 daughter merozoites. Merozoites are liberated into the circulation by rupture of the host red blood cell and reinitiate the cycle of invasion and multiplication. P. vivax preferentially invades human reticulocytes (8), immature red blood cells, if they are positive for the Duffy blood group antigen (9), a receptor for a family of chemokines (10); in contrast, P. falciparum invades the much more plentiful mature erythrocytes. Two proteins (PvRBP-1 and PvRBP-2) located at the apical pole of P. vivax merozoites specifically adhere to reticulocytes (11). The concentration of reticulocytes in normal, peripheral blood (0.5–1.5%) is not sufficient to sustain P. vivax in vitro, even with periodic replenishment. Therefore, to successfully recycle generations of P. vivax in vitro, it is necessary to add to cultures elevated concentrations of Duffy-positive reticulocytes. In the most successful previous attempt at in vitro cultivation, Mons et al. (12) used reticulocyte-enriched blood obtained from monkeys that were treated with a hemolytic drug, phenylhydrazine hydrochloride, to cycle P. vivax in a continuous shaker culture; however, parasite densities, after initially increasing, then rapidly decreased and parasites disappeared by the 6th cycle (12). Human reticulocyte preparations separated from whole blood using a Percoll Renograffin gradient also failed to support in vitro cultures of P. vivax (13). In this report, we describe a method that does allow continuous cycling of high density, infective parasites in human erythrocytes.
MATERIALS AND METHODS
P. Vivax.
The Chesson (New Guinea) strain was isolated shortly after World War II from an American soldier (14) and first passaged to splenectomized Aotus monkeys from an infected human volunteer in 1969 (15). Cryopreserved parasites (sample AI 671; 9,720 parasites per μl; 5/11/94) were obtained from the Malaria Branch, Division of Parasitic Diseases, National Center for Infectious Diseases, Centers for Disease Control and Prevention (Atlanta). This strain has been maintained in Aotus monkeys at the Walter Reed Army Institute of Research since August 1994.
Animal Welfare.
All husbandry and animal research practices were performed in keeping with approved standards of the Walter Reed Army Institute of Research, an Association for the Assessment and Accreditation of Laboratory Animal Care, International accredited facility, and in accordance with the Guide for the Care and Use of Laboratory Animals (16).
Aotus (Owl) Monkeys.
Two karyotypes of Aotus monkeys were used: Aotus nancymai (karyotype 1, Peruvian origin) and Aotus lemurinus griseimembra (karyotype II, Colombian origin). All nonimmune animals were splenectomized prior to inoculation with cryopreserved or cultured parasites.
Inoculation of Monkeys with Parasites and Monitoring of Infection.
An A. nancymai monkey (WR 2110) was infected by intravenous injection with Chesson strain P. vivax parasites. Cryopreserved, infected blood was thawed in saline processing solutions (12% NaCl/1.6% NaCl/0.2% Dextrose plus 0.9% NaCl; Baxter Health Care), resuspended to a volume of 0.5 ml in McCoy’s 5A medium modified with L-glutamine (219.2 mg/liter) with 25 mM Hepes buffer (M5AMM, GIBCO/BRL) supplemented with 20% human AB+ serum, and injected into the saphenous vein using a Butterfly ST infusion set (25 × 3/8, 3 and 1/2 inch tubing, Abbott Hospitals, North Chicago, IL) attached to the syringe containing parasites. Starting on day 5 after inoculation of parasites, and every second day until the monkey either self-cured or was treated with chloroquine HCl, a volume of 0.1 ml of blood was collected to monitor the parasitemia, reticulocytosis, and hematocrit (HCT). Infected blood was collected on days 17 and 21 post infection to initiate in vitro cultures. A second owl monkey, A. griseimembra (WR421), was inoculated with predominately ring and young trophozoite stage parasites obtained from cycle 6 of culture 593. A volume of 0.25 ml of cultured parasites (6% HCT) containing 3,100 parasites per μl was inoculated into the saphenous vein as described above. Monitoring of the infection was as previously described. On day 24 after inoculation of cultured parasites, the monkey received a single dose of chloroquine (5 mg/ml) and parasites cleared but were observed again on day 52. Blood from this monkey was collected on day 64, prior to chloroquine retreatment, to initiate cultures used to compare the ability of fresh and cryopreserved reticulocytes to support the growth of parasites.
Collection of Blood and Initiation of Cultures.
Monkey blood was collected for in vitro culturing when 0.1–0.5% of the erythrocytes were infected and approximately 70% of the parasites were rings or young trophozoites. The infected blood was collected into a Vacutainer tube (3.0 ml draw, Becton Dickinson) containing citrate phosphate dextrose adenine anticoagulant solution (0.15 ml for every 0.5 ml of blood collected). The cap of the Vacutainer tube was removed and the contents diluted 1:5 with M5AMM. To remove leukocytes, the diluent was collected under gas pressure (5% CO2/5% O2/90% N2) through a loosely packed CF 11 cellulose powder column prewetted with medium (17). M5AMM (0.5 ml) was used to wash the column, while permitting the gas flow to continue until bubbles appeared at the bottom of the column. The effluent was centrifuged [500 × g, for 10 min at room temperature (RT)], washed first in M5AMM, then rewashed in M5AMM supplemented with 20% AB+ human serum. Thick and thin Giemsa-stained blood films were examined to reconfirm parasite stage and density. Static candle jar cultures (6% HCT, 500 μl per well) were initiated in 4-well culture plates (NUNCLON, Nunc) in a candle jar (5). After 20–24 hr the culture medium was changed. At the time when 50% of the parasites had begun schizogony (2–4 nuclei), usually by 38 hr, the shaker phase was initiated. The culture was centrifuged (500 × g, 10 min, RT), and the medium aspirated to approximately 6 mm above the pellet. Blood enriched up to 20% reticulocytes by differential centrifugation in homologous plasma was warmed to 37°C and mixed with the infected cell pellet. The reticulocyte volume added was equivalent to the calculated volume of packed, cultured cells, resulting in a 2-fold increase in the amount of blood in culture with each generation of parasites. Because reticulocytes mature to erythrocytes within 24 hr in culture, they had to be replenished during schizogony with each cycle of parasites. Fresh M5AMM with 20% AB+ human serum was added to obtain a 12–15% HCT, and the suspension was transferred to a Type T Tissue Culture flask (Bellco Glass), gassed for 15 sec with 5% CO2/5% O2/90% N2, and shaken 100 cycles per min at 37°C for 10–12 hr. The contents of the Type T Tissue Culture flask were then centrifuged (500 × g, 10 min, RT), the supernatant removed, and thick and thin Giemsa-stained slides made from the pellet. The presence of rings or young trophozoite stage parasites confirmed that parasites had invaded the reticulocytes. Three counts of 104 cells were done from different areas of the same thin film slide to determine parasite densities. To repeat the cycle, fresh medium was added to the pellet to bring the HCT to 6%, and cultures were returned to the candle jar. In an experiment to determine if parasites invaded and differentiated to merozoites in cryopreserved reticulocytes, the aforementioned procedure was slightly altered: reticulocytes were added 40–44 hr after initiation of the cultures and parasites counted on 1 μl thick films.
Preparation of Reticulocytes.
Reticulocytes were separated from the Duffy antigen positive blood of a patient undergoing therapy for hemochromatosis. After 3–6 weeks of repeated therapeutic phlebotomy, the concentration of reticulocytes in the peripheral blood (3.0–5.0%) of these patients often is elevated to 3–10 times that of the reticulocyte concentration (0.5–1.0%) of normal adults. This factor, in addition to the relatively long treatment period for some patients (1–2 years) and the weekly availability of units (450 ml) of blood, made therapeutic blood a reliable source from which to prepare enriched reticulocytes.
A modified method of enriching reticulocytes by differential centrifugation in homologous plasma (18) was used. White cells were removed from chilled blood (4°C, 30 min) using a leukocyte separation filter (Sepacell, Baxter Health Care). The filtered blood was aliquoted among ten 50-ml conical centrifuge tubes, centrifuged (1,000 × g, 15 min, 22–24°C), and the volume of packed cells was recorded. Excess plasma was removed from each tube to achieve an 80–85% HCT. The excess plasma was stored at 4°C and used for a second centrifugation step (described below). Warmed blood (1 hr, 37°C) was centrifuged (35,000 × g, 30 min, 32°C) and the plasma aspirated to approximately 6 mm above the top of the pellet. The remaining plasma and the upper 25% of the pellet were removed and pooled. This suspension was then diluted with an equal volume of fresh, homologous plasma (50% HCT). The 37°C incubation, centrifugation, and aspiration procedures were repeated except that only the top 10% of the final pellets were collected and pooled. For a unit of blood, this resulted in a final packed volume of approximately 10–12 ml of reticulocytes enriched 15–20%. Reticulocytes were washed 2 times with 10 ml of M5AMM. The pellet was stored at 4°C for up to 2 weeks in an equivalent volume of M5AMM without loss of viability. Prior to the addition to P. vivax cultures, the pellet was rewashed with fresh M5AMM, centrifuged to pack the cells, and warmed to 37°C. Alternatively, reticulocytes were cryopreserved in Glycerolyte 57 solution (Baxter Health Care).
Analysis of rRNA Sequence of Cultured Parasites.
Genomic DNA was prepared from two human (P. vivax and P. falciparum) and two monkey (P. cynomolgi and P. knowlesi) malarias. Two strains of P. vivax (Chesson and Salvador), were obtained from the American Type Culture Collection. Approximately 0.2 ml of packed blood cells recovered from the American Type Culture Collection and cultured parasites was dissolved in 5 vol of DNAzol (GIBCO/BRL) and extracted with 1 vol of phenol/chloroform (24:24, vol/vol). The aqueous phase was precipitated with an equal volume of isopropanol and resuspended in 40 μl water. Total RNA of the parasites was recovered by the method of Li et al. (19) from the blood smears prepared during cultivation. Amplification of 18S rRNA sequence was carried out by primer 841 (sense: GAACGAGATCTTAACCTGC) and primer 844 (antisense: TAITGATAAAGATTACCTA). Single-stranded cDNA was synthesized by reverse transcriptase and then amplified using the same conditions for DNA PCR (19). The resulting products were separated by electrophoresis on a 1.5% agarose gel and the transferred blot was hybridized with a 32P-labeled oligoprobe specific for the P. vivax rRNA expressed in blood stages (probe 741: AACCGAATTCAGTCCCACGT).
RESULTS
P. vivax parasites were passaged successfully from owl monkey to human erythrocytes and recycled in culture for 6–8 generations four times by periodic addition of human reticulocytes during schizogony, thus providing merozoites with the target cells needed for reinvasion (data shown from three experiments). Parasite densities remained stable, after an initial increase, until cultures were arbitrarily terminated between 10–14 days, at which time parasites were cryopreserved. The number of parasites present in 104 erythrocytes 10–12 hr after each addition of reticulocytes was determined for two cultures initiated on different days from the same monkey (Table 1). In both cultures, the parasite density increased at least 2-fold by cycle 3 and generally remained stable for the remainder of the time the parasites were in culture.
Table 1.
P. vivax densities in continuous culture at 10–12 hr after the addition of reticulocytes
| Cycle | Subcultures | Hours in culture | Dilution of monkey to human blood | Mean no. of parasites in 104 cells (n = 3) | SE |
|---|---|---|---|---|---|
| Culture 593 | |||||
| 1 | Primary | 0 | 1:0 | 14.00 | 1.15 |
| 2 | a | 48 | 1:1 | 15.67 | 2.03 |
| 3 | a* | 97 | 1:2 | 28.00 | 2.52 |
| 4 | b | 145 | 1:4 | 42.00 | 3.61 |
| c* | 145 | 1:4 | 33.00 | 1.53 | |
| 5 | b† | 194 | 1:8 | 28.00 | 1.73 |
| d | 194 | 1:8 | 28.00 | 1.73 | |
| e | 194 | 1:8 | 34.33 | 2.03 | |
| 6 | d* | 242 | 1:16 | 34.67 | 4.91 |
| e‡ | 242 | 1:16 | 25.67 | 1.67 | |
| 7 | f | 291 | 1:32 | 32.00 | 1.00 |
| g† | 291 | 1:32 | 32.67 | 0.33 | |
| 8 | f† | 339 | 1:64 | 33.00 | 0.58 |
| Culture 194 | |||||
| 1 | Primary | 0 | 1:0 | 21.67 | 1.20 |
| 2 | a | 51 | 1:1 | 43.00 | 3.00 |
| 3 | a* | 99 | 1:2 | 46.00 | 2.65 |
| 4 | b | 147 | 1:4 | 44.67 | 1.67 |
| c | 147 | 1:4 | 54.67 | 2.03 | |
| 5 | b | 195 | 1:8 | 56.67 | 1.86 |
| c† | 195 | 1:8 | 50.33 | 0.88 | |
| 6 | b† | 245 | 1:16 | 55.00 | 3.06 |
The primary cultures from an owl monkey were passaged to human reticulocytes beginning the first subculture (a) or the second generation of parasites.
As reticulocytes were added and the volume of parasitized cells increased, subcultures were divided at the beginning of some cycles and were named alphabetically.
Subculture was removed and cryopreserved.
Subculture was removed and injected into monkey.
Because the volume of erythrocytes doubled each cycle with the addition of reticulocytes, a stable parasite density was equivalent to at least a doubling of parasite numbers every 48 hr. For example, the volume of packed erythrocytes from primary culture 593 was approximately 80 μl, while the volume of packed cells at the beginning of cycle 4 was approximately 450 μl and contained one monkey to four human erythrocytes. As the volume of cells in culture increased, subcultures were made, starting at cycle 4 (Table 1). The addition of reticulocytes decreased the parasite density by 50% during schizogony; however, by the beginning of the next cycle, they had returned to or exceeded the previous density. Since added blood contained 15–20% reticulocytes, it appears that the availability of 7.5–10% reticulocytes in cultures during the 10–12 hr that schizonts rupture is sufficient to maintain a stable parasite density.
Successful timing for adding reticulocytes to the culture depended on initiating cultures from predominately ring-infected erythrocytes obtained from the blood of owl monkeys having synchronous infections. Cultured parasites had a generation cycle of approximately 48 hr, the same as in vivo. Cultures remained fairly synchronous for the entire time of in vitro cultivation, with additions of reticulocytes routinely made between 36–40 hr after each new generation of parasites began development in a static candle jar. When the total parasite counts for all cycles in the two cultures were combined and sorted by developmental stages present 10–12 hr after the addition of reticulocytes, over 80% of the parasites were found to be rings and less than 3% were schizonts (Table 2), an indication of rapid synchronized invasion of new cells. No gametocytes, the sexual forms of the parasite, were observed at any time.
Table 2.
Frequency distribution of P. vivax developmental stages at 10–12 hr after the addition of reticulocytes
| Stage | Culture
|
|
|---|---|---|
| 593 | 194 | |
| Ring | 948 ( 83.01) | 964 (85.77) |
| Young trophozoite | 160 ( 14.01) | 144 (12.81) |
| Mature trophozoite | 5 (0.44) | 0 (0) |
| Immature schizont | 23 (2.01) | 9 (0.80) |
| Mature schizont | 6 (0.53) | 7 (0.62) |
| Total | 1142 (100.00) | 1124 (100.00) |
Pooled from all generational cycles shown in Table 1. Numbers in parentheses represent percent of total number of parasites counted.
Cryopreserved reticulocytes were compared with fresh reticulocytes for their ability to support invasion and maintain parasites. Before fresh or cryopreserved reticulocytes were added, cultures contained predominantly schizonts (Table 3). Minimal recycling of parasites occurred before the addition of reticulocytes and agitation of the cultures, as evidenced by the low proportion of ring forms, 18.3% for cryopreserved and 29.4% for fresh reticulocytes. After the addition of fresh or cryopreserved reticulocytes, over 80% of the parasites in both cultures were rings, demonstrating that cryopreserved reticulocytes were as capable of supporting growth and invasion as were fresh cells.
Table 3.
Comparison of the number and developmental stages of P. vivax using fresh or cryopreserved reticulocytes
| Fresh reticulocytes
|
Cryopreserved reticulocytes
|
|||
|---|---|---|---|---|
| Before addition (cycle 4) | After addition (cycle 5) | Before addition (cycle 4) | After addition (cycle 5) | |
| Ring | 98 (29.83) | 661 (83.99) | 67 (18.31) | 541 (84.66) |
| Trophozoite | 53 (15.92) | 66 (8.39) | 80 (21.86) | 21 (3.29) |
| Schizont | 182 (54.65) | 60 (7.62) | 219 (58.83) | 77 (12.05) |
| Total | 333 (100.00) | 787 (100.00) | 366 (100.00) | 639 (100.00) |
The numbers of parasites compared were those present in a 1 μl volume thick film. Numbers in parentheses represent the percent of total number of parasites counted.
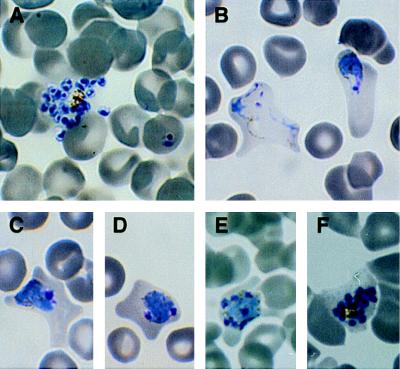
While the morphology of cultured parasites appeared typical of the asexual forms of P. vivax seen in monkeys and humans, the infected erythrocytes containing older trophozoites and schizonts appeared larger and more distorted (Fig. 1 B–F). Trophozoites appeared highly ameboid (Fig. 1B) and began nuclear division by 32–36 hr (Fig. 1 D–F). Despite the enlargement and distortion of infected erythrocytes, their cell membranes remained intact until mature schizonts, typically containing between 16 and 24 merozoites, ruptured (Fig. 1A). Although Schuffner’s stippling was evident in Giemsa-stained-infected erythrocytes from monkey, it was not observed in cultured parasites.
Figure 1.
Asexual stages of P. vivax cultured parasites. (A) Mature schizont releasing merozoites and a ring stage parasite, (B) young, highly ameboid, maturing trophozoite, (C) mature trophozoite in a distorted erythrocyte, (D) immature schizont containing two nuclei, (E) multinucleated schizont, (F) mature schizont in an intact erythrocyte. (×990.)
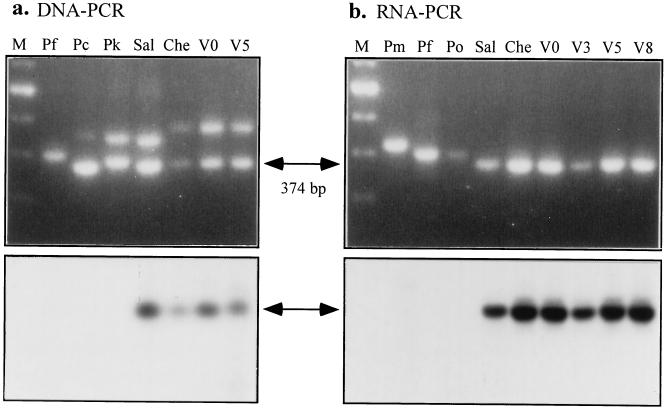
Species confirmation of the cultivated parasites was done by analyzing rRNA genes and their transcripts. A partial sequence of the 18S rRNA was amplified from genomic DNA using a pair of primers (containing hypervariable regions conserved in the genus Plasmodium); this yielded two genes (20, 21), one expressed only in the blood stages (A form) and one only in the mosquito (C form). P. vivax Chesson strain differs from P. vivax Salvador, P. falciparum, and two monkey malarias, P. cynomolgi and P. knowlesi, in the relative size of these genes (Fig. 2a Upper); the two amplified gene products of P. falciparum were the same size and appeared as a single band. The parasites collected from the donor monkey before cultivation (V0) and after five complete cycles of cultivation (V5) shared an identical rRNA gene pattern to the Chesson strain supplied by the American Type Culture Collection. Reverse transcriptase–PCR of cultured parasites from cycles 3, 5, and 8 (culture 593) produced only C genes, which were identical in size to the donor V0 and type Chesson (Fig. 2b). These PCR products were about 375 nt, smaller in size than P. malariae (420 nt), P. falciparum and P. ovale (400 nt) (19). The identity of the species was further confirmed by blot hybridization of transferred PCR products with 32P-labeled oligonucleotide probe complimentary to the C form of the rRNA gene (Fig. 2 a and b Lower).
Figure 2.
Confirmation of cultured parasite P. vivax Chesson strain using characteristics of the ribsomal 18S RNA gene. (a Upper) Amplification products for a Plasmodium specific region of the 18S rRNA gene. The two bands represent the sporozoite (top) and blood stage (bottom) forms of the gene. Type specimen Chesson strain (Che) can be clearly distinguished not only from P. falciparum (Pf), P. cynomolgi (Pc), and P. knowlesi (Pk), but from the Central American strain of P. vivax (Sal). The type specimen is identical to monkey reared parasites at the beginning of culture (V0) and after five cycles (V5). (Lower) Southern blot of the seven samples with an oligo probe specific for the blood stage gene of P. vivax. Size standards are shown in M (100 bp ladder). (b Upper) Amplification profiles of cDNA derived from rRNA by reverse transcriptase–PCR for two additional primate species, P. malariae (Pm) and P. ovale (Po), as well as for culture cycles 3 (V3), 5 (V5), and 8 (V8). Southern blots with the same probe as in a are shown below.
The infectivity of cultured parasites was demonstrated by injecting predominantly ring/young trophozoite stage parasites from cycle 6 of culture 593 (3,100 parasites per μl) into an Aotus monkey (WR421). Nine days later, parasites were first observed on thin films from its peripheral blood. The prepatent period and densities were comparable to those observed in monkeys infected directly from uncultured, cryopreserved blood.
DISCUSSION
By alternating between a static candle jar and shaker culture and by periodically adding reticulocytes, P. vivax was maintained in culture for 6–8 cycles with rising, then stable, densities. In all cases, cultures were terminated arbitrarily at about 2 weeks; there was no indication that they could not be continued indefinitely. The total number of parasites in culture increased at least 2-fold with each generation of parasites. The rate of propagation was limited by the proportion of cells that were reticulocytes at the time of merozoite invasion, which, in our cultures, never exceeded 10%. If it were possible to replace all erythrocytes with reticulocytes, then the increase in P. vivax would probably equal that observed during P. falciparum cultivation. Nevertheless, the parasite count of predominantly ring and young trophozoites at the 6th cycle in culture 194 was approximately 7,200 parasites per μl, equivalent to that reported for P. vivax (5.33 × 103- 1 × 104 parasites per μl) from patients who had failed drug treatment (3, 22) and much higher than that of most semi-immune individuals living in endemic areas (23).
The presence of only Chesson strain P. vivax in cultures was confirmed by analysis of the 18S rRNA gene, a highly sensitive and specific indicator for Plasmodium (24). The cultured parasites shared an identical PCR product banding pattern for the 18S rRNA genes with type Chesson, which was distinct from the Salvador strain, two monkey malarias, and cultured P. falciparum. Analysis of RNA prior to cultivation (V0) and from cycles 3, 5, and 8 also confirmed that only Chesson was present throughout cultivation, and that only the A, or blood stage gene, was being expressed.
In earlier reported attempts to cultivate P. vivax, both Mons et al. (12) and Lanners (13) reported that densities decreased because of the apparent fragility of parasitized cells, rupture of schizonts before maturation, and the inability of merozoites to invade reticulocytes. Our culture conditions supported both invasion of reticulocytes and maintenance of parasites in cells that did not rupture prior to schizont maturity. Possibly, crucial modifications were the replacement of RPMI medium 1640 with M5AMM, use of a higher concentration of human serum (20% rather than 10%), and maintenance of parasites under static candle jar conditions until nuclear divisions began. Brockelman et al. (25) first demonstrated complete schizogony of P. vivax isolates incubated under candle jar conditions; both Mons et al. (12) and Lanners (13) maintained P. vivax suspension cultures with continual mixing or agitation. In our cultures, agitation occurred only for 10–12 hr after nuclear divisions began and reticulocytes were added; this served to maximize contact between parasites and target cells. Once invasion of erythrocytes occurred, ring and young trophozoite stage parasites were transferred back to a static culture for growth and differentiation. We found that invasion was enhanced when cultures where mixed as first demonstrated by Mons et al. (12), but unlike Mons (26) we did not find that this reduced the number of schizonts that matured.
The method of reticulocyte preparation may affect the ability of merozoites to bind and invade reticulocytes. Lanners (13), adding reticulocytes prepared from a Percoll Renograffin gradient to cultures, found that merozoites adhered to, but did not invade erythrocytes. Similarly, we found that preparations, containing 60% reticulocytes prepared from Percoll Renograffin gradients, did not support invasion, whereas preparations containing 20% reticulocytes prepared by differential centrifugation in homologous plasma did. Reticulocyte preparations from Percoll gradients have been used successfully in P. vivax invasion studies (11, 27), suggesting that Renograffin may interfere with invasion. Although Barnwell et al. (27) reported culture parasite densities of 10–15%, based on counts of ring stages present 8–10 hr after the addition of Percoll-prepared reticulocytes, they apparently maintained the culture only until invasion was demonstrated. Perhaps preparation of reticulocytes in plasma supplies or preserves essential components for receptors and membrane stability. That cryopreserved reticulocytes supported invasion and growth of parasites as well as freshly prepared reticulocytes should increase the convenience of this method.
Now that stable in vitro recycling of asexual parasite generations has been achieved, attention can be given to several limitations of the system. First, the lack of gametocyte formation in the cultures prohibits their use for infecting mosquitoes. This may be a characteristic of our Chesson strain, which had not been passed through mosquitoes for 3 years and rarely produced gametocytes in infected monkeys. Second, the procedure is labor intensive and follows an inconvenient schedule that requires attention every 12 hr at midnight on alternate days. It was because of our inability to follow this schedule for prolonged periods that cultures were not maintained beyond eight cycles. We are currently trying to adjust the parasite cycle to coincide with more convenient hours. Third, the requirement for high reticulocyte counts prior to enrichment may limit this procedure to investigators with access to facilities that routinely treat individuals for hemochromatosis. The use of frozen reticulocytes, which we have shown is feasible, should reduce this problem. Additionally, the use of culture systems in which hematopoietic cells proliferate and differentiate to mature erythrocytes (28) are currently being investigated as a source of reticulocytes for cultivation. We hope this in vitro method will provide a basic research tool, that much like the cultivation technique for P. falciparum, will advance the understanding of parasite biochemistry, immunology, molecular biology, physiology, and pharmacology.
Acknowledgments
We sincerely thank William Collins at the Centers for Disease Control and Prevention (Chamblee, GA) for providing the cryopreserved Chesson strain parasites that we passaged to monkeys; Thomas F. McCutchan at the National Institute of Allergy and Infectious Diseases (Bethesda) for support in the molecular identification of the parasites; Ted Hadfield at the Department of Microbiology, Armed Forces Institute of Pathology (Washington, DC) for photographing the parasites; the staff of the Department of Transfusional Medicine at the National Institutes of Health (Bethesda) for providing blood for reticulocyte enrichment; Michael Junio and Jennifer Lavelle for technical assistance with the monkeys; and Imogene Schneider for advice and encouragement.
ABBREVIATIONS
- M5AMM
McCoy’s 5A medium modified with L-glutamine with 25 mM Hepes buffer
- HCT
hematocrit
- RT
room temperature
References
- 1.Anonymous WHO Weekly Epidemiol Rec. 1994;42:309–314. [Google Scholar]
- 2.Rieckman K H, Davis D R, Hutton D C. Lancet. 1989;ii:1183–1184. doi: 10.1016/s0140-6736(89)91792-3. [DOI] [PubMed] [Google Scholar]
- 3.Myat-Phone-Kyaw, Myint-Oo, Myint-Lwin Thaw-Zin, Kyin-Hla-Aye, Nwe-Nwe-Yin Trans R Soc Trop Med Hyg. 1993;87:687. doi: 10.1016/0035-9203(93)90294-z. [DOI] [PubMed] [Google Scholar]
- 4.Baird J K, Nalim M F, Basri H, Masbar S, Leksana B, Tjitra E, Dewi R M, Wignall F S. Trans R Soc Trop Med Hyg. 1996;90:409–411. doi: 10.1016/s0035-9203(96)90526-x. [DOI] [PubMed] [Google Scholar]
- 5.Trager W, Jensen J B. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen-Dinh P, Campbell C C, Collins W E. Science. 1980;209:1249–1251. doi: 10.1126/science.6773146. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen-Dinh P, Gardner A L, Campbell C C, Skinner J C, Collins W E. Science. 1981;212:1146–1148. doi: 10.1126/science.7233207. [DOI] [PubMed] [Google Scholar]
- 8.Kitchen S F. Am J Trop Med. 1939;18:347–359. [Google Scholar]
- 9.Miller L H, Mason S J, Clyde D F, McGinnis M H. N Engl J Med. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 10.Horuk R, Chitnis C E, Darbonne W C, Colby T J, Rybicki A, Hadley T J, Miller L H. Science. 1993;261:1182–1184. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- 11.Galinski M R, Medina C C, Ingravallo P, Barnwell J W. Cell. 1992;69:1213–1226. doi: 10.1016/0092-8674(92)90642-p. [DOI] [PubMed] [Google Scholar]
- 12.Mons B, Collins W E, Skinner J C, van der Star W, Croon J J, van der Kaay H J. Exp Parasitol. 1988;66:183–188. doi: 10.1016/0014-4894(88)90089-6. [DOI] [PubMed] [Google Scholar]
- 13.Lanners H N. Parasitol Res. 1992;78:699–701. doi: 10.1007/BF00931524. [DOI] [PubMed] [Google Scholar]
- 14.Ehrman F C, Ellis J M, Young M D. Science. 1945;101:377. doi: 10.1126/science.101.2624.377. [DOI] [PubMed] [Google Scholar]
- 15.Ward R A, Rutledge L C, Hickman R L. Nature (London) 1969;224:1126–1127. doi: 10.1038/2241126a0. [DOI] [PubMed] [Google Scholar]
- 16.National Institutes of Health (1985) Guide for the Care and Use of Laboratory Animals, NIH Publication 86–23 (Natl. Inst. Health, Bethesda).
- 17.Williams S G, Richards W H G. Ann Trop Med Parasitol. 1973;67:169–178. [PubMed] [Google Scholar]
- 18.Murphy J R. J Lab Clin Med. 1973;82:334–341. [PubMed] [Google Scholar]
- 19.Li J, Wirtz R A, McConkey G A, Sattabongkot J, Waters A P, Rogers M J, McCutchan T H. Exp Parasitol. 1995;18:182–190. doi: 10.1006/expr.1995.1107. [DOI] [PubMed] [Google Scholar]
- 20.Gunderson J H, Sogin M L, Wollett G, Hollingdale M, De La Cruz V F, Waters A P, McCutchan T H. Science. 1987;238:933–937. doi: 10.1126/science.3672135. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Wirtz R A, McConkey G A, Sattabongkot J, McCutchan T H. Mol Biochem Parasitol. 1994;65:283–289. doi: 10.1016/0166-6851(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz I K, Lackritz E M, Patchen L C. N Engl J Med. 1991;324:927. doi: 10.1056/NEJM199103283241317. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg R, Andre R G, Ngampatom S, Hatz C, Burge R. Trans R Soc Trop Med Hyg. 1990;84:14–21. doi: 10.1016/0035-9203(90)90366-m. [DOI] [PubMed] [Google Scholar]
- 24.McCutchan T F, Li J, McConkey J A, Rogers M J, Waters A P. Parasitol Today. 1995;11:134–138. doi: 10.1016/0169-4758(95)80132-4. [DOI] [PubMed] [Google Scholar]
- 25.Brockelman C R, Peerapan T-A, Laovanitch R. J Protozool. 1985;32:76–80. doi: 10.1111/j.1550-7408.1985.tb03016.x. [DOI] [PubMed] [Google Scholar]
- 26.Mons B. Blood Cells. 1990;16:299–312. [PubMed] [Google Scholar]
- 27.Barnwell J W, Nichols M E, Rubinstein P. J Exp Med. 1989;169:1795–1802. doi: 10.1084/jem.169.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAdams T A, Miller W M, Papoutsakis E T. Bio/Technology. 1996;14:341–349. doi: 10.1016/0167-7799(96)10047-0. [DOI] [PubMed] [Google Scholar]