Abstract
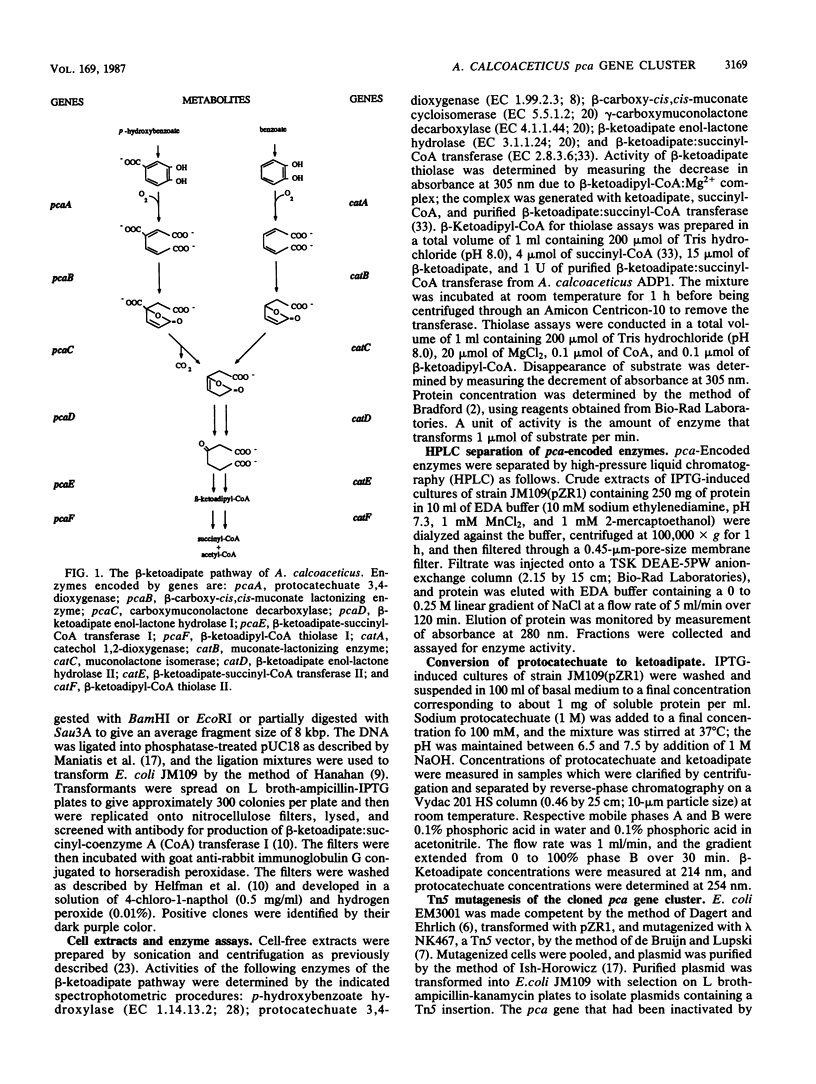
The beta-ketoadipate pathway of Acinetobacter calcoaceticus comprises two parallel metabolic branches. One branch, mediated by six enzymes encoded by the cat genes, converts catechol to succinate and acetyl coenzyme A (acetyl-CoA); the other branch, catalyzed by products of the pca genes, converts protocatechuate to succinate and acetyl-CoA by six metabolic reactions analogous or identical to those of the catechol sequence. We used the expression plasmid pUC18 to construct expression libraries of DNA from an A. calcoaceticus mutant strain from which the cat genes had been deleted. Immunological screening with antiserum to the pcaE gene product, beta-ketoadipate:succinyl-CoA transferase I, resulted in the isolation of a cloned 11-kilobase-pair (kbp) fragment which inducibly expressed all six pca genes under control of the lac promoter on pUC18. The induced Escherichia coli cells formed the six pca gene products at levels 10- to 30-fold higher than found in fully induced A. calcoaceticus cultures, although protocatechuate 3,4-dioxygenase (the iron-containing product of the pcaA gene) from the recombinant strain possessed a relatively low turnover number. An E. coli culture expressing the cloned pca genes quantitatively converted protocatechuate to beta-ketoadipate; failure of the organism to metabolize the latter compound can be most readily ascribed to relatively low pool levels of succinyl-CoA, a required substrate for beta-ketoadipate:succinyl-CoA transferase, in E. coli. The gene order and direction of transcription were determined to be pcACBDFE by identification of enzymes expressed in subclones, by using natural transformation to identify subclones carrying DNA corresponding to dysfunctional alleles in mutant A. calcoaceticus strains, and by restriction mapping of both the 11-kbp fragment and derivatives of the 11-kbp fragment containing Tn5 in the pcaA, pcaB, pcaD, and pcaE genes. The fragment containing the pca gene hybridized strongly and specifically to a previously cloned fragment containing A. calcoaceticus cat genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Wheelis M. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 2. The role of protocatechuate as inducer. Eur J Biochem. 1968 Jan;3(3):293–304. doi: 10.1111/j.1432-1033.1968.tb19529.x. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Doten R. C., Gregg L. A., Ornston L. N. Influence of the catBCE sequence on the phenotypic reversion of a pcaE mutation in Acinetobacter calcoaceticus. J Bacteriol. 1987 Jul;169(7):3175–3180. doi: 10.1128/jb.169.7.3175-3180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham D. R., Stirling L. A., Ornston L. N., Perry J. J. Intergeneric evolutionary homology revealed by the study of protocatechuate 3,4-dioxygenase from Azotobacter vinelandii. Biochemistry. 1980 Jan 8;19(1):149–155. doi: 10.1021/bi00542a023. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Lillard M. O., Schwartz R. D. Protocatechuate 3, 4-dioxygenase from Acinetobacter calcoaceticus. Biochemistry. 1976 Feb 10;15(3):582–588. doi: 10.1021/bi00648a020. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Consequences of reduced intracellular coenzyme A content in Escherichia coli. J Bacteriol. 1986 Jun;166(3):866–871. doi: 10.1128/jb.166.3.866-871.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E. L., Ornston L. N. Cloning and expression of Acinetobacter calcoaceticus catechol 1,2-dioxygenase structural gene catA in Escherichia coli. J Bacteriol. 1986 Nov;168(2):815–820. doi: 10.1128/jb.168.2.815-820.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. II. Enzymes of the protocatechuate pathway. J Biol Chem. 1966 Aug 25;241(16):3787–3794. [PubMed] [Google Scholar]
- Patel R. N., Mazumdar S., Ornston L. N. Beta-ketoadipate enol-lactone hydrolases I and II from Acinetobacter calcoaceticus. J Biol Chem. 1975 Aug 25;250(16):6567–6567. [PubMed] [Google Scholar]
- Shanley M. S., Neidle E. L., Parales R. E., Ornston L. N. Cloning and expression of Acinetobacter calcoaceticus catBCDE genes in Pseudomonas putida and Escherichia coli. J Bacteriol. 1986 Feb;165(2):557–563. doi: 10.1128/jb.165.2.557-563.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. T., Finnerty W. R. Insertional specificity of transposon Tn5 in Acinetobacter sp. J Bacteriol. 1984 Feb;157(2):607–611. doi: 10.1128/jb.157.2.607-611.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sramek S. J., Frerman F. E. Purification and properties of Escherichia coli coenzyme A-transferase. Arch Biochem Biophys. 1975 Nov;171(1):14–26. doi: 10.1016/0003-9861(75)90002-8. [DOI] [PubMed] [Google Scholar]
- Wijnands R. A., Müller F. A study of p-hydroxybenzoate hydroxylase from Pseudomonas fluorescens: chemical modification of histidine residues. Biochemistry. 1982 Dec 21;21(26):6639–6646. doi: 10.1021/bi00269a005. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yeh W. K., Fletcher P., Ornston L. N. Evolutionary divergence of co-selected beta-ketoadipate enol-lactone hydrolases in Acinetobacter calcoaceticus. J Biol Chem. 1980 Jul 10;255(13):6342–6346. [PubMed] [Google Scholar]
- Yeh W. K., Fletcher P., Ornston N. Homologies in the NH2-terminal amino acid sequences of gamma-carboxymuconolactone decarboxylases and muconolactone isomerases. J Biol Chem. 1980 Jul 10;255(13):6347–6354. [PubMed] [Google Scholar]
- Yeh W. K., Ornston L. N. Evolutionarily homologous alpha 2 beta 2 oligomeric structures in beta-ketoadipate succinyl-CoA transferases from Acinetobacter calcoaceticus and Pseudomonas putida. J Biol Chem. 1981 Feb 25;256(4):1565–1569. [PubMed] [Google Scholar]
- Yeh W. K., Ornston L. N. Origins of metabolic diversity: substitution of homologous sequences into genes for enzymes with different catalytic activities. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5365–5369. doi: 10.1073/pnas.77.9.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh W. K., Shih C., Ornston L. N. Overlapping evolutionary affinities revealed by comparison of amino acid compositions. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3794–3797. doi: 10.1073/pnas.79.12.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]




