Abstract
The carabid beetle Galerita lecontei has a pair of abdominal defensive glands that secrete a mixture of formic acid, acetic acid, and lipophilic components (long-chain hydrocarbons and esters). Formic acid, at the concentration of 80%, is the principal constituent. The beetle ejects the secretion as a spray, which it aims accurately toward parts of the body subjected to assault. At full capacity, the glands store 4.5 mg of formic acid (3% of body mass), enough for upward of six ejections. The beetle reloads the glands at a rate of 126 μg of formic acid per day. For the approximately 500 secretory cells of the glands, this means an hourly output of 10 ng of formic acid per cell, or about 5% of cell volume. Replenishing empty glands to their full formic acid load takes the beetle an estimated 37 days. Replenishing the 0.7 mg of formic acid expended in a single discharge takes 5.5 days.
Keywords: predation, chemical defense, secretory rate, Carabidae
The noted British naturalist John Wray, in what must be one of the earliest references to insect chemistry (1), called attention to the production of an acid “juyce” by ants. Such fluid, containing formic acid, is well known nowadays to be ejected by ants of the subfamily Formicinae. Formic acid is a potent irritant, deterrent to vertebrates and invertebrates alike, and it serves ants effectively in defense (2). Not surprisingly, the capacity to produce the compound has evolved in other insects as well, notably in carabid beetles (3). We report here on one carabid, Galerita lecontei, that ejects a spray containing formic acid at the concentration of 80%. We describe the glands that produce the fluid, give details of the chemical composition of the liquid, and as part of an attempt to obtain some measure of the defensive “budget” of the beetle, provide an estimate of the rate at which formic acid is produced by the secretory cells of the glands.
The study was prompted by preliminary observations by one of us (T.E.) on both G. lecontei and its morphologically very similar congener Galerita janus. Both beetles appeared to discharge formic acid, because they invariably came to reek characteristically of the compound when picked up by hand in the field. It was also clear that both beetles ejected the acid at high concentration, because the discharged fluid failed to turn filter paper impregnated with cobaltous chloride from blue to pink, indicating that it was relatively water-free. The data presented here were obtained almost exclusively with G. lecontei. Where obtained also with G. janus, it is so indicated.
MATERIALS AND METHODS
Statistics.
Values are presented throughout as mean ± SD.
The Beetles.
G. lecontei were taken in ultraviolet light traps in spring, on the grounds of the Archbold Biological Station, near Lake Placid, Highlands County, FL. The few G. janus that were also used were collected under rocks near streams in Ithaca, Tompkins County, NY. The beetles were kept in groups in containers with soil, and maintained for weeks on freshly cut up mealworms (larvae of Tenebrio molitor) and water. Body mass of G. lecontei males (n = 10) and females (n = 4) was found to be 156 ± 18 mg and 159 ± 27 mg, respectively.
Gland Anatomy.
For gross anatomical study of the glands, beetles were dissected under saline solution. For scanning electronmicroscopy, gland parts were fixed in alcoholic Bouin’s solution, dehydrated in ethanol, and critical-point dried. For phase microscopy, freshly dissected clusters of secretory cells of the glands were directly mounted in saline solution on microscope slides. Isolation of the cuticular duct system characteristically associated with these cells was effected by treating gland cell clusters overnight with 10% potassium hydroxide.
Directionality of Spray.
To assess the beetles’ ability to aim their spray, individuals were affixed by the pronotum to a metal rod with a droplet of wax, then positioned in normal stance on a sheet of red indicator paper (filter paper soaked in alkaline phenolphthalein solution). The beetles were then caused to discharge by pinching individual appendages with forceps. When discharges occurred, these became registered as white spray patterns on the filter paper. To keep the beetles from discharging prematurely when being affixed to the rod, they were cooled beforehand for some minutes by refrigeration.
Duration of Spray.
This parameter was assessed from a 16-mm film taken at 270 frames per second of a single G. lecontei, in left lateral view, discharging twice in succession in response to pinching of the left foreleg. The film was converted to video format and analyzed frame-by-frame by videotape playback.
Chemistry.
Mass spectra were obtained with a Hewlett-Packard (HP) 5890 gas chromatograph [25 m × 0.25 mm fused-silica capillary column coated with HP-5 (5% phenyl methylsilicone) stationary phase (0.25 μm film thickness)], coupled to a HP 5971 Mass Selective Detector. Oven temperature conditions were consistent throughout: 35°C for 4 min, increased to 260°C at 10°C/min. Introduction of samples (in dichloromethane solution) into the chromatograph was by splitless injection.
Net determinations of formic acid were effected by the colorimetric technique of Lang and Lang (4), modified in that samples were heated to 55°C for 30 min before absorbance measurement.
Secretion samples for analysis were obtained either as whole sac extracts (storage sacs of glands excised intact and crushed under solvent), or as pure secretion samples (secretion taken up from lumen of excised gland sacs with a glass micropipette, as in Fig. 1C).
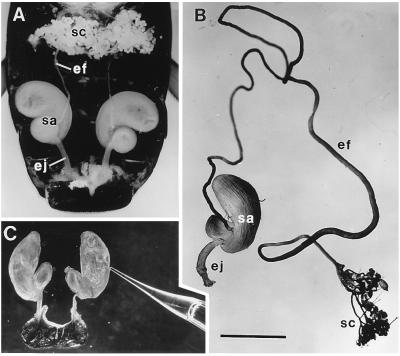
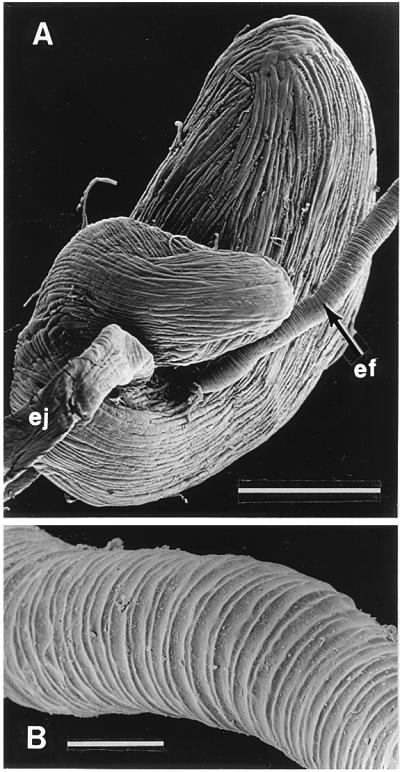
Figure 1.
(A) Dorsal view of abdominal cavity of G. janus, showing the two defensive glands. Other abdominal organs have been largely excised. Each gland consists of an aggregate of secretory cells (sc), an efferent duct (ef), a storage sac (sa), and an ejaculatory duct (ej). (B) Isolated gland of G. lecontei (labels as in A). (C) Excised glandular sacs of G. lecontei, being “milked” of secretion; one of the sacs has been pierced with a glass micropipette, which is taking up secretion by capillary action. (Bar: 2 mm.)
RESULTS
The Glands.
The defensive glands of G. lecontei (like those of G. janus) form an identical pair of structures, lying side by side in the abdominal cavity. Ordinarily concealed by fat body, gut, and reproductive organs, the glands are easily exposed by dissection (Fig. 1A). Each gland consists of a dense aggregate of secretory cells, an efferent duct that drains these, a kidney-shaped sac in which secretion is stored, and an ejaculatory duct through which the secretion is discharged. The ejaculatory ducts open marginally near the abdominal tip to the sides of the anus. The cellular aggregates of the two glands are sometimes closely apposed (Fig. 1A). The efferent ducts, which are ordinarily much coiled, are of substantial length (Fig. 1B). The storage sacs are thickly enveloped by compressor muscles, clearly revealed in scanning electronmicrographs (Fig. 2A). The efferent ducts are devoid of muscles, flexible, and resistant to compression; their outer surface is characteristically sculpted (Fig. 2B).
Figure 2.
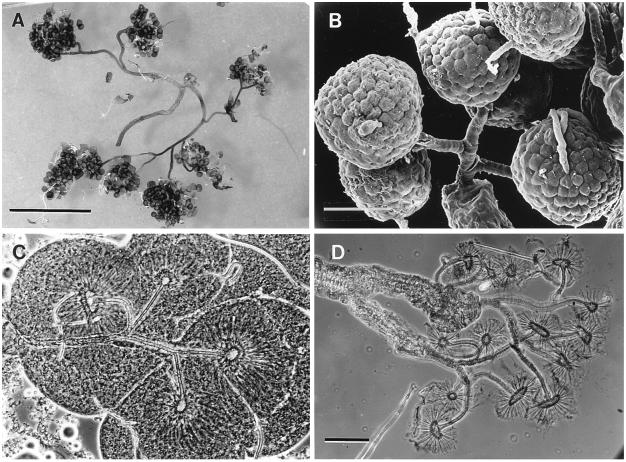
G. lecontei: Scanning electronmicrographs. (A) Storage sac of gland, showing the investiture of compressor muscles (labels as in Fig. 1A). (B) Detail of efferent duct (the sculpting is of the cuticular wall; the duct lacks intrinsic muscles). (Bars: A, 0.5 mm; B, 50 μm.)
Each secretory cell aggregate, when teased apart, is revealed to consist of a number of cell clusters, linked by drainage tubes that merge to form the efferent duct (Fig. 3A). Close-up examination of the secretory cells shows each to have its own drainage tubule, arising from a distinct, characteristically star-shaped, intracellular organelle (Fig. 3 B and C). The tubules and organelles are cuticular and could be isolated readily by potassium hydroxide treatment of the cells (Fig. 3D).
Figure 3.
G. lecontei. (A) Teased-out secretory cell aggregate, showing the clusters of cells and their respective drainage tubes, which converge to form the efferent duct (not shown). (B) Closeup view of a group of secretory cells, joined by their individual drainage tubules (scanning electronmicrograph). (C) Group of secretory cells (fresh unstained mount; phase micrograph) showing the star-shaped cuticular organelles and their drainage tubules. (D) Same as preceding (treated with potassium hydroxide), showing the isolated organelles and tubules. (Bars: A, 2 mm; B and D, 50 μm.)
The secretory cells of G. lecontei are large and can be easily counted under low microscopic magnification. Cell counts (left aggregate/right aggregate) for six beetles were found to be: males (253/243)(252/230) (267/273)(238/245)(253/241); female (257/289). Total number of secretory cells per beetle are thus 507 ± 29. Measurements from scanning electronmicrographs gave a secretory cell diameter of 71 ± 6 μm (n = 19). Cell volume is therefore on average 187 × 10−6 μl.
Directionality of Spray.
The tethered beetles (G. lecontei and G. janus) responded to pinching of appendages by discharging accurately aimed ejections. Individual legs were always precisely targeted (Fig. 4 A and B), as were antennae when these were stimulated. How the beetles achieve aiming remains unclear, although close observation suggested that downward deflection of the abdominal tip was involved. The ejections were always unilateral, from the gland of the side of the appendage stimulated (beetles that ceased to respond by spraying after repeated stimulation of the legs of one side, still sprayed, albeit contralaterally, when subsequently stimulated by pinching the legs of the opposite side). The total number of discharges that could be elicited from individual beetles varied. For six G. lecontei that had been kept undisturbed for 3.5 months beforehand that total was 6.5 ± 1.5 (range = 5 to 9) discharges per beetle.
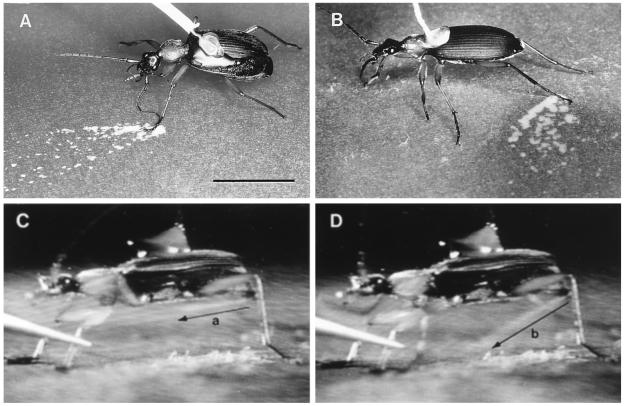
Figure 4.
G. lecontei. (A) Spray pattern on indicator paper elicited by pinching a beetle’s left midleg with forceps. (B) Comparable to preceding, but elicited by pinching another beetle’s left hindleg. (C and D) Frames from a slow-motion videotape of a beetle’s discharge (discharge duration = 116 ms). The jet of spray, visible as a faint line above the arrow in each picture, changes in direction during the ejection. The arrows a and b denote the limits of this directional sweep. (Bar: 1 cm.)
Duration of Discharges.
Temporal analysis of the videotape gave 116 ms and 80 ms for the duration of the two consecutive discharges elicited from the beetle. Fluid was seen to emerge from the abdominal tip as a continuous narrow jet, which oscillated in direction during the course of the discharge. The oscillation ensured that the stimulated leg was hit by the spray. Fig. 4 C and D shows the limits of the directional sweep (arrows a and b) undergone by the jet during the discharge. In the first ejection, the jet emerged in direction a, and underwent the cycle a/b/a a total of 3.5 times. In the shorter second ejection, the jet also emerged in direction a, but underwent the a/b/a cycle only 1.5 times.
Chemistry (G. lecontei).
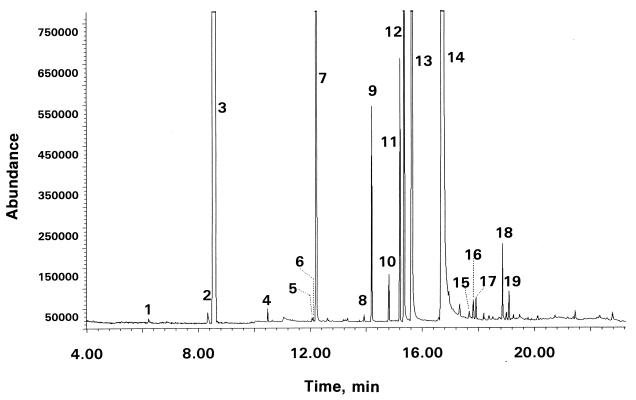
Characterization of components. Gas chromatographic/mass spectral examination of an extract of the secretion (whole glandular sacs from two males and two females) revealed presence of formic acid, acetic acid, and 19 lipophilic components. The two acids were characterized by the mass spectra of their pentafluorobenzyl esters (5). The lipophilic components (Table 1, Fig. 5) were characterized by their mass spectra; the position of the double bond in the three alkenes (1-nonene, 5-undecene, 4-undecene) was determined from mass spectra of their dimethyl disulfide derivatives (5). All spectral characterizations were confirmed by comparison with published mass spectra (6).
Table 1.
Lipophilic components of G. lecontei secretion
| Peak label* | Compound | Relative amount |
|---|---|---|
| 1 | Octane | 0.02 |
| 2 | 1-Nonene | 0.07 |
| 3 | Nonane | 33.13 |
| 4 | Decane | 0.04 |
| 5 | 5-Undecene | 0.15 |
| 6 | 4-Undecene | 0.15 |
| 7 | Undecane | 4.54 |
| 8 | Octyl acetate | 0.20 |
| 9 | Nonyl formate | 0.63 |
| 10 | 1-Decanol | 0.15 |
| 11 | Tridecane | 0.73 |
| 12 | Nonyl acetate | 3.81 |
| 13 | Decyl formate | 10.06 |
| 14 | Decyl acetate | 45.64 |
| 15 | Nonyl butyrate | 0.30 |
| 16 | Decyl propionate | 0.08 |
| 17 | Undecyl acetate | 0.07 |
| 18 | Decyl butyrate | 0.22 |
| 19 | Dodecyl acetate | 0.09 |
Peak labels are those designated in Fig. 5
Figure 5.
A reconstructed ion chromatogram of volatiles in a dichloromethane extract of defensive secretion of a male G. lecontei. Peak labels correspond to numbers in Table 1.
Relative ratio of components.
For the lipophilic components these ratios could be determined by comparison of chromatographic peak areas. Five separate secretion samples (pure secretion from two males and three females) were chromatographed for the purpose. There was no qualitative, and little quantitative, variation in the composition of the samples. Table 1 gives the values obtained with one of the male samples.
In the conversion of the carboxylic acids to pentafluorobenzyl esters, decyl acetate, the principal lipophilic component, remains unchanged. It was possible therefore to calculate (from a mixed sample of secretion of males and females), by chromatographic peak comparisons, the relative ratio of formic acid to acetic acid and to decyl acetate in the secretion. That ratio was found to be 100:1:5. In relation to total lipophilic material (which amounts to about twice the quantity of decyl acetate) that ratio can be taken to be 100:1:10.
Concentration of formic acid.
Weighed samples of secretion were collected from the glands of three males and three females. These samples were of pure secretion obtained from excised glandular sacs with a micropipette. Fluid mass was determined by weighing the micropipette before and after fluid uptake. For each sample, secretion was obtained from both sacs. Analyses provided net values of formic acid per sample. Concentrations were calculated from these values.
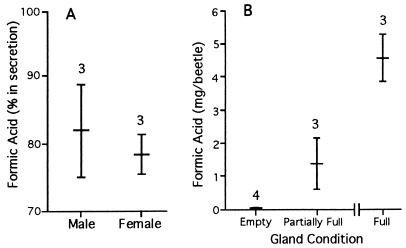
The results, given in Fig. 6A, show that formic acid is produced at a concentration of about 80% by both sexes.
Figure 6.
(A) Percent formic acid in defensive secretion of male and female G. lecontei. (B) Formic acid content of G. lecontei glandular sacs, plotted as a function of time since the glands were last “milked” to depletion. Beetles were milked 0 days (empty) and 11 days (partially full) beforehand, or were kept unmilked since captured 3.5 months earlier (full glands). Numbers above bars give sample sizes.
Composition of secretion.
The value of 80% for formic acid, taken in conjunction with the relative ratios given under b above, provides a basis for estimating the actual composition of the secretion (Table 2). About 90% of the secretion is accounted for by that estimate. We presume the uncharacterized remaining fraction to be water.
Table 2.
Composition of G. lecontei secretion
| Component | Percent |
|---|---|
| Formic acid | 80 |
| Acetic acid | 1 |
| Lipophilic components | 8 |
| Water (?) | 11 |
The relatively high content of lipophilic material suggests that the secretion should be biphasic. Indeed, on the numerous occasions when secretion was taken up into micropipettes, the fluid was always noted to consist of a clear outer phase, and an equally clear, coarsely dispersed (and presumably lipoidal) inner phase.
Net quantity of formic acid per beetle.
Three beetles of known mass (two females, one male) that had been caged undisturbed for 3.5 months after capture, were dissected and their glandular sacs analyzed whole for formic acid content (both sacs were lumped per sample). Such individuals could be expected to have replete glands, and their glandular sacs did indeed appear to be maximally distended when excised. Beetle body mass was 148 ± 17 mg. Glandular formic acid content (Fig. 6B, full glands) was 4.56 ± 0.72 mg per beetle, or about 3% of beetle body mass.
Rate of formic acid production.
To obtain a measure of this parameter, two sets of glandular sacs were analyzed for formic acid content, one from beetles (n = 4 males) that had just been caused to spray to depletion, the other from beetles (n = 3 males) that were caused to spray to depletion 11 days beforehand and had since been kept undisturbed. To cause the latter beetles to exhaust their secretion, they were held by hand over indicator paper and pinched with forceps until they ceased to respond by spraying. Sacs were again excised whole and analyzed as a lumped pair per sample. The results (Fig. 6B) show that the beetles do indeed expell virtually their entire secretion when they spray to exhaustion, and that they reload their glands relatively slowly. After 11 days the sacs contained on average 1.39 mg of formic acid, or about 30% of the mean maximal load. The beetles produced formic acid at a rate, on average, of 126 μg per day.
DISCUSSION
G. lecontei is not unique among arthropods in having the capacity to produce a potent toxicant at high concentration for defense. The whip scorpion, Mastigoproctus giganteus, sprays a mixture containing 84% acetic acid (7), and certain cockroaches (Platyzosteria spp.) discharge a secretion containing 95% 2-ethyl acrolein (8). Nor is the beetle unusual among Carabidae in being chemically protected. Carabids as a group have defensive glands that are essentially similar, and presumed to be homologous, to those of G. lecontei (9). Diverse chemicals have been characterized from the defensive glands of Carabidae, including quinones, phenols, aldehydes, acids, esters, and hydrocarbons (3). Formic acid itself is produced by a number of species (3), at concentrations said to be high in some cases (10, 11).
The ability to aim the spray is shared by G. lecontei with other insects that discharge formic acid-containing secretions, including formicine ants (2, 12) and notodontid caterpillars (13). Among Carabidae, all species that have been studied in detail, whether they discharge formic acid or entirely different toxicants, direct their ejections (14–21). Tests with a number of carabids have shown their defensive sprays to be effectively deterrent to predators (14–16).
The presence of lipophilic components in acidic defensive secretions of arthropods is not unusual. Such “additives” are believed to function primarily as wetting and penetration-promoting agents (7), but there is evidence that they are intrinsically deterrent as well, at least vis á vis some predators. Simple hydrocarbons, for instance, can be topically irritating to insects (22). The primary active principle in the secretion of G. lecontei is doubtless formic acid, but the lipophilic constituents could be more than mere surfactants.
Insect gland cells characteristically have intracellular cuticular organelles and drainage tubules comparable to those present in the secretory cells of G. lecontei. These organelles vary considerably in structure (23), but given their general occurrence are presumed to be fundamentally involved in the secretory process (24, 25). We found only a single type of secretory cell in the G. lecontei glands and presume these cells to produce both the acidic and lipophilic components of the secretion.
Our quantitative data provide a basis for estimating some fundamental parameters pertinent to the G. lecontei defense. Given that the glands, at full capacity, hold 4.5 mg of formic acid, and that the beetles can spray 6.5 times before exhausting their supply, single ejections must contain, on average, 0.7 mg of formic acid. If one assumes that the ejections we elicited from beetles by pinching with forceps were of normal magnitude, then 0.7 mg could be the average minimal quantity of formic acid expended by G. lecontei to counter an attack.
The beetles replenish their glands at a rate of 126 μg of formic acid per day. This means that it takes the beetles 36 days to restore empty glands to full capacity, and over 5 days to replenish the 0.7 mg of formic acid lost in a single ejection.
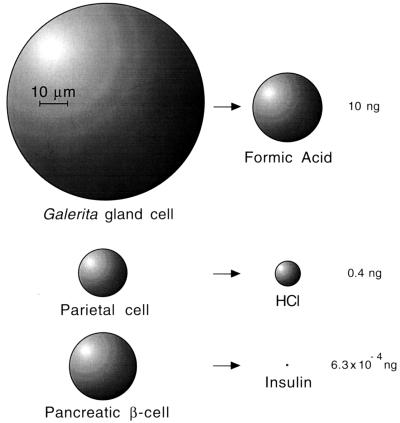
Although the overall rate of glandular replenishment is slow, the secretory output of the individual cells is relatively copious. The approximately 500 secretory cells possessed by the beetle secrete formic acid at the rate of 10 ng per cell per hr. Assuming a density of 1 for the fluid, this amounts to approximately 5% (10 × 10−6 μl) of cell volume per hr, or 1.2 times the cell volume per day. In terms of net amount of formic acid produced, this exceeds the rate of hydrochloric acid production by human parietal cells, and (by far, as expected) the rate of hormone production by an endocrine cell (Fig. 7).
Figure 7.
Comparison of hourly secretory output of G. lecontei gland cells, human parietal cells, and human pancreatic β-cells. Values for the latter two cell types were calculated from data in the literature (27–29). All cells are to scale shown. Spheres denoting secretion droplets are based on density = 1 for each substance.
It seems likely that formic acid in G. lecontei is produced, as it is in ants (26), from the amino acids l-serine and glycine, via N5-formyltetrahydrofolate. G. lecontei, being carnivorous, is certain to obtain both these precursors as staples with its diet. If we assume, somewhat arbitrarily, that the protein in the beetle’s diet contains 5% each of l-serine and glycine, then about 60 mg of protein would be required by the beetle to provision its glands to capacity with formic acid. For the beetle to refurbish the 0.7 mg of formic acid expended in a single discharge would demand 9 mg of protein, while synthesizing formic acid at the observed rate would require 1.6 mg of protein per day, or 1% of beetle body mass. Defense, evidently, does not come “free” for G. lecontei.
Acknowledgments
This is paper no. 146 in the series Defense Mechanisms of Arthropods; paper no. 145 is ref. 30. We thank the staff of the Archbold Biological Station, Lake Placid, FL, for kindnesses extended to us during our stay at the station; Nick Upton and Kevin Flay of Green Umbrella Limited, Bristol, England, for taking the high-speed motion picture film; and Mark Deyrup for comments on the manuscript. The study was supported by Grants AI02908 and GM53830 from the National Institutes of Health.
References
- 1.Wray J. Philos Trans R Soc London. 1670;68:2063–2069. [Google Scholar]
- 2.Holldöbler B, Wilson E O. The Ants. Cambridge, MA: Harvard Univ. Press; 1990. [Google Scholar]
- 3.Weatherston J, Percy J E. In: Handbook of Experimental Pharmacology: Arthropod Venoms. Bettini S, editor. Vol. 48. New York: Springer–Verlag; 1978. pp. 511–554. [Google Scholar]
- 4.Lang E, Lang H. Frezenius Z Anal Chem. 1972;260:8–10. [Google Scholar]
- 5.Attygalle A B, Morgan E D. Anal Chem. 1986;58:3054–3058. [Google Scholar]
- 6.McLafferty F W, Stauffer D B. The Wiley/NBS Registry of Mass Spectral Data. New York: Wiley; 1989. [Google Scholar]
- 7.Eisner T, Meinwald J, Monro A, Ghent R. J Insect Physiol. 1961;6:272–298. [Google Scholar]
- 8.Waterhouse D F, Wallbank B E. J Insect Physiol. 1967;13:1657–1669. doi: 10.1016/0022-1910(67)90161-8. [DOI] [PubMed] [Google Scholar]
- 9.Forsyth D J. Trans Zool Soc London. 1972;32:249–309. [Google Scholar]
- 10.McCullogh T. Ann Entomol Soc Am. 1967;60:861. [Google Scholar]
- 11.Scott P D, Hepburn H R, Crewe R M. Insect Biochem. 1975;5:805–811. [Google Scholar]
- 12.Eisner T, Baldwin I T, Conner J. Proc Natl Acad Sci USA. 1993;90:6716–6720. doi: 10.1073/pnas.90.14.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attygalle A B, Smedley S R, Meinwald J, Eisner T. J Chem Ecol. 1993;19:2089–2104. doi: 10.1007/BF00979649. [DOI] [PubMed] [Google Scholar]
- 14.Eisner T. J Insect Physiol. 1958;2:215–220. [Google Scholar]
- 15.Eisner T, Swithenbank C, Meinwald J. Ann Entomol Soc Am. 1963;56:37–41. [Google Scholar]
- 16.Eisner T, Hurst J J, Meinwald J. Psyche. 1963;70:94–116. [Google Scholar]
- 17.Eisner T, Meinwald Y C, Alsop D W, Carrel J E. Ann Entomol Soc Am. 1968;61:610–613. [Google Scholar]
- 18.Aneshansley D J, Eisner T, Widom J M, Widom B. Science. 1969;165:61–63. doi: 10.1126/science.165.3888.61. [DOI] [PubMed] [Google Scholar]
- 19.Eisner T, Aneshansley D J. Science. 1982;215:83–85. doi: 10.1126/science.215.4528.83. [DOI] [PubMed] [Google Scholar]
- 20.Eisner T, Attygalle A B, Eisner M, Aneshansley D J, Meinwald J. Chemoecology. 1991;2:29–34. [Google Scholar]
- 21.Attygalle A B, Meinwald J, Eisner T. J Chem Ecol. 1992;18:489–498. doi: 10.1007/BF00994247. [DOI] [PubMed] [Google Scholar]
- 22.Peschke K, Eisner T. J Comp Physiol A. 1987;161:377–388. doi: 10.1007/BF00603963. [DOI] [PubMed] [Google Scholar]
- 23.Noirot C, Quennedey A. Ann Rev Entomol. 1974;19:61–80. [Google Scholar]
- 24.Eisner T, McHenry F, Salpeter M M. J Morphol. 1964;115:355–399. doi: 10.1002/jmor.1051150304. [DOI] [PubMed] [Google Scholar]
- 25.Happ G M. J Insect Physiol. 1968;14:1821–1837. [Google Scholar]
- 26.Hefetz A, Blum M S. Biochim Biophys Acta. 1978;543:484–496. doi: 10.1016/0304-4165(78)90303-3. [DOI] [PubMed] [Google Scholar]
- 27.Helander H F. In: Ultrastructure of the Digestive Tract. Motta P M, Fujita H, Correr S, editors. Boston: Martinus Nijhoff; 1988. pp. 35–51. [Google Scholar]
- 28.Johnson L R. In: Gastrointestinal Physiology. 4th Ed. Johnson L R, editor. St. Louis: Mosby; 1991. pp. 66–84. [Google Scholar]
- 29.Taborsky G J., Jr . In: Textbook of Physiology. 21st Ed. Patton H D, Fuchs A F, Hille B, Sher A M, Steiner R, editors. Philadelphia: Saunders; 1989. pp. 1522–1533. [Google Scholar]
- 30.Radford, P., Attygalle, A. B., Meinwald, J., Smedley, S. R. & Eisner, T. (1997) J. Nat. Prod., in press. [DOI] [PubMed]