Abstract
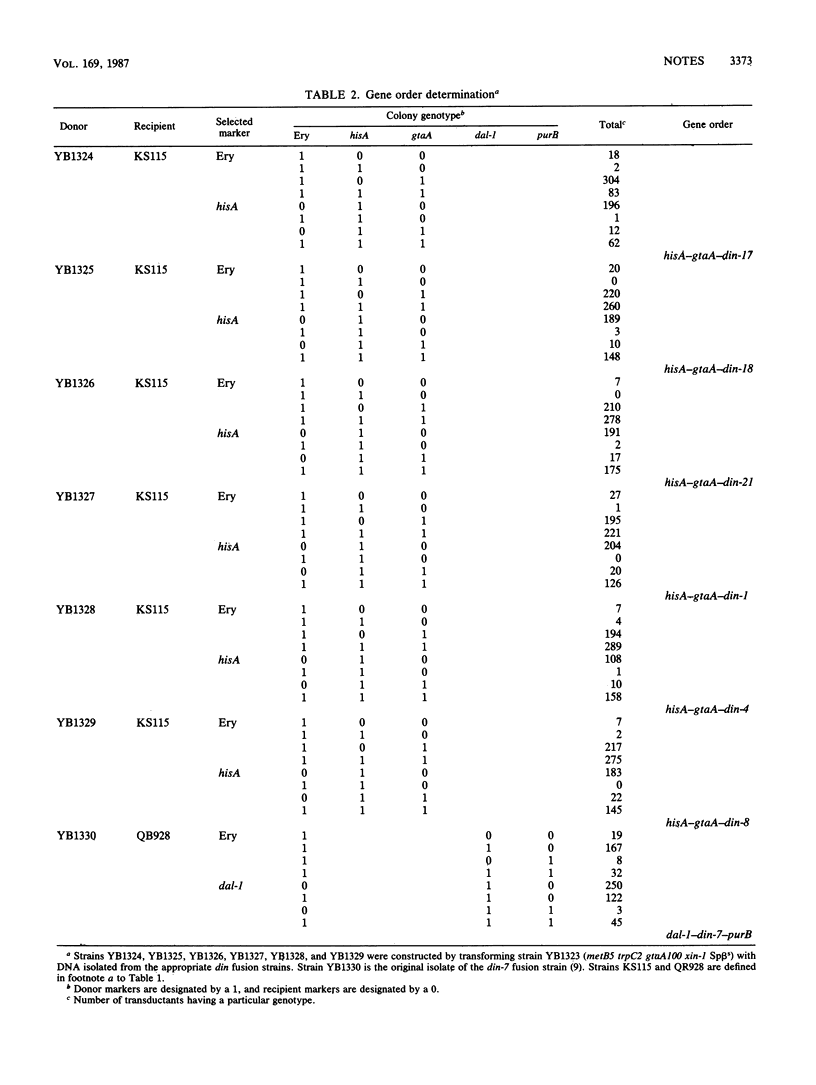
Previously isolated DNA damage-inducible (din) genes of Bacillus subtilis have been mapped on the bacterial chromosome by bacteriophage PBS1-mediated transduction. The din genes have been localized to three positions on the B. subtilis map. dinA cotransduction with the hisA locus was 80%, while dinC cotransduction with this marker was about 56%. dinB is unlinked to hisA, but its cotransduction with the dal-1 and purB loci was 84 and 22%, respectively.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagg A., Kenyon C. J., Walker G. C. Inducibility of a gene product required for UV and chemical mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5749–5753. doi: 10.1073/pnas.78.9.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogliano M., Schendel P. F. Evidence for the inducibility of the uvrB operon. Nature. 1981 Jan 15;289(5794):196–198. doi: 10.1038/289196a0. [DOI] [PubMed] [Google Scholar]
- Hoch J. A., Anagnostopoulos C. Chromosomal location and properties of radiation sensitivity mutations in Bacillus subtilis. J Bacteriol. 1970 Aug;103(2):295–301. doi: 10.1128/jb.103.2.295-301.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. Expression of the E. coli uvrA gene is inducible. Nature. 1981 Feb 26;289(5800):808–810. doi: 10.1038/289808a0. [DOI] [PubMed] [Google Scholar]
- Lepesant-Kejzlarová J., Lepesant J. A., Walle J., Billault A., Dedonder R. Revision of the linkage map of Bacillus subtilis 168: indications for circularity of the chromosome. J Bacteriol. 1975 Mar;121(3):823–834. doi: 10.1128/jb.121.3.823-834.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Love P. E., Lyle M. J., Yasbin R. E. DNA-damage-inducible (din) loci are transcriptionally activated in competent Bacillus subtilis. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6201–6205. doi: 10.1073/pnas.82.18.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love P. E., Yasbin R. E. Genetic characterization of the inducible SOS-like system of Bacillus subtilis. J Bacteriol. 1984 Dec;160(3):910–920. doi: 10.1128/jb.160.3.910-920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love P. E., Yasbin R. E. Induction of the Bacillus subtilis SOS-like response by Escherichia coli RecA protein. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5204–5208. doi: 10.1073/pnas.83.14.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kane C., Stephens M. A., McConnell D. Integrable alpha-amylase plasmid for generating random transcriptional fusions in Bacillus subtilis. J Bacteriol. 1986 Nov;168(2):973–981. doi: 10.1128/jb.168.2.973-981.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins J. B., Youngman P. J. Construction and properties of Tn917-lac, a transposon derivative that mediates transcriptional gene fusions in Bacillus subtilis. Proc Natl Acad Sci U S A. 1986 Jan;83(1):140–144. doi: 10.1073/pnas.83.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky E. M., Roberts J. W. Kinetics of RecA protein-directed inactivation of repressors of phage lambda and phage P22. J Mol Biol. 1980 May 25;139(3):319–328. doi: 10.1016/0022-2836(80)90133-3. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Hoch J. A. Revised genetic linkage map of Bacillus subtilis. Microbiol Rev. 1985 Jun;49(2):158–179. doi: 10.1128/mr.49.2.158-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E. DNA repair in Bacillus subtilis. I. The presence of an inducible system. Mol Gen Genet. 1977 Jun 8;153(2):211–218. [PubMed] [Google Scholar]
- Yasbin R. E. DNA repair in Bacillus subtilis. II. Activation of the inducible system in competent bacteria. Mol Gen Genet. 1977 Jun 8;153(2):219–225. [PubMed] [Google Scholar]
- Youngman P., Zuber P., Perkins J. B., Sandman K., Igo M., Losick R. New ways to study developmental genes in spore-forming bacteria. Science. 1985 Apr 19;228(4697):285–291. doi: 10.1126/science.228.4697.285. [DOI] [PubMed] [Google Scholar]


