Abstract
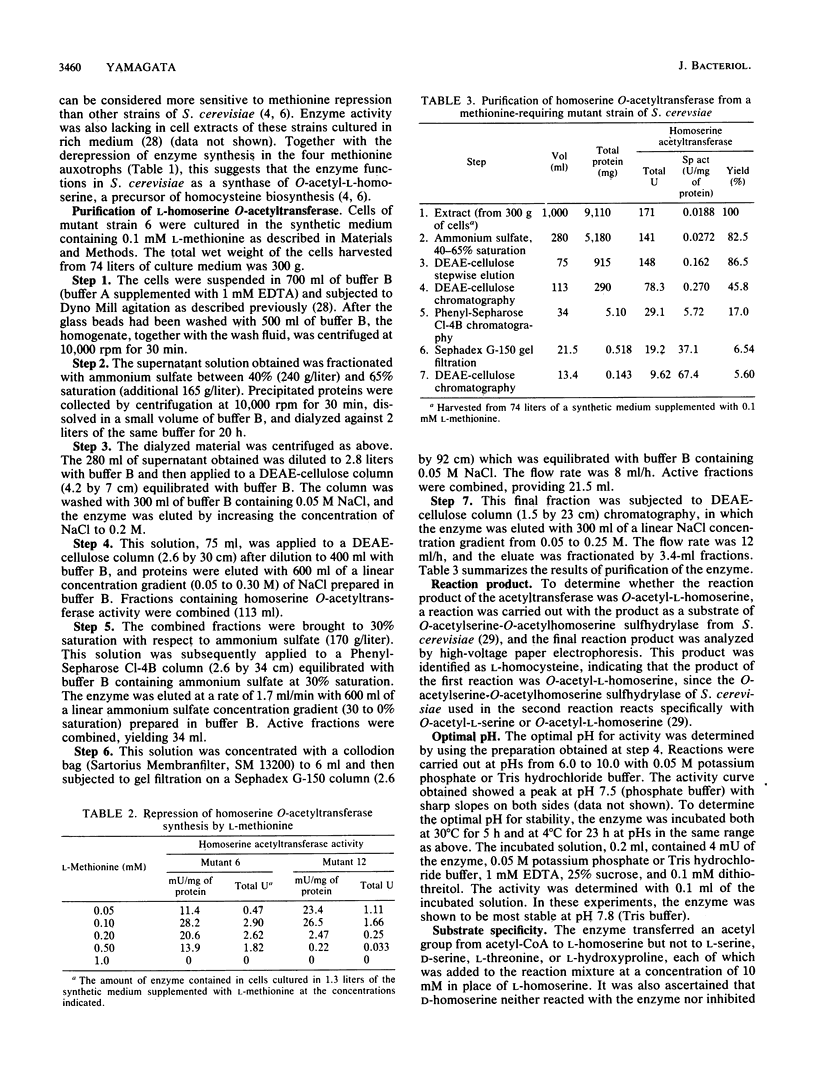
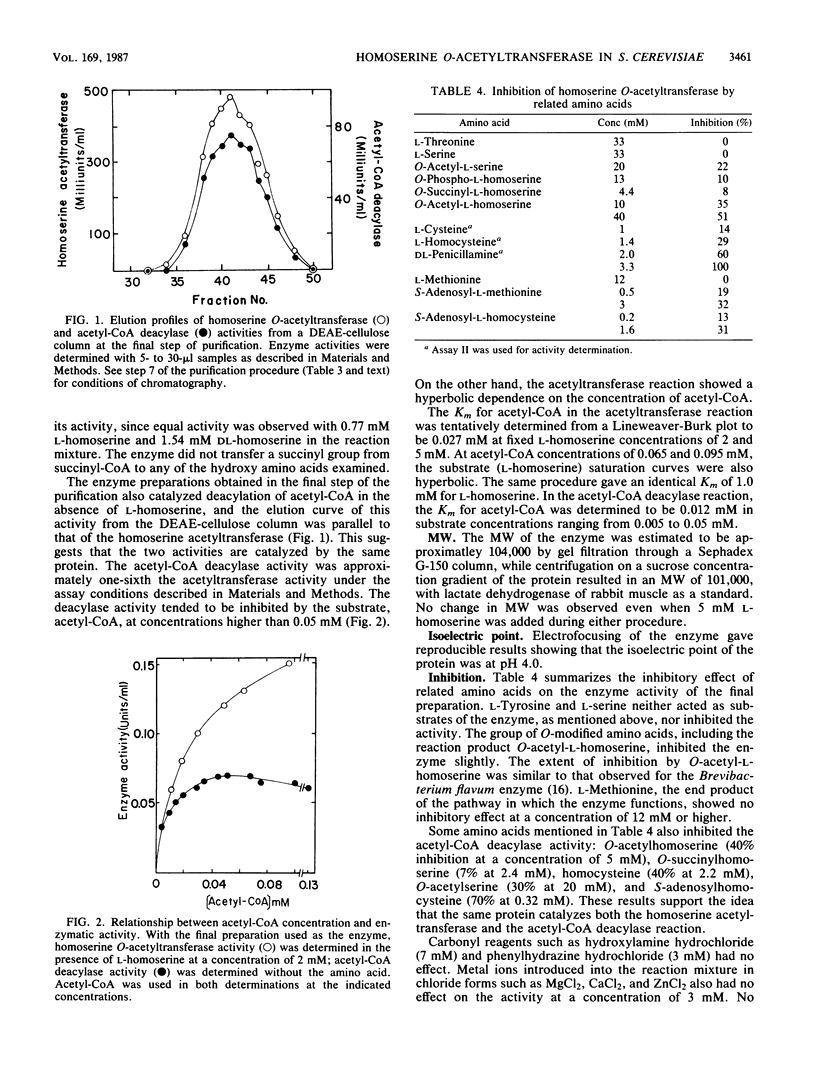
A wild-type strain and six methionine auxotrophs of Saccharomyces cerevisiae were cultured in a synthetic medium supplemented with 0.1 mM L-cysteine or L-methionine and analyzed for the synthesis of homoserine O-acetyltransferase (EC 2.3.1.31). Among them, four mutant strains exhibited enzyme activity in cell extracts. Methionine added to the synthetic medium at concentrations higher than 0.1 mM repressed enzyme synthesis in two of these strains. The enzyme was partially purified (3,500-fold) from an extract of a mutant strain through ammonium sulfate fractionation and chromatography on columns of DEAE-cellulose, Phenyl-Sepharose C1-4B, and Sephadex G-150. The enzyme exhibited optimal pH at 7.5 for activity and at 7.8 for stability. The reaction product was ascertained to be O-acetyl-L-homoserine by confirming that it produced L-homocysteine in an O-acetyl-L-homoserine sulfhydrylase reaction. The Km for L-homoserine was 1.0 mM, and for acetyl coenzyme A it was 0.027 mM. The molecular weight of the enzyme was estimated to be approximately 104,000 by Sephadex G-150 column chromatography and 101,000 by sucrose density gradient centrifugation. The isoelectric point was at pH 4.0. Of the hydroxy amino acids examined, the enzyme showed reactivity only to L-homoserine. Succinyl coenzyme A was not an acyl donor. In the absence of L-homoserine, acetyl coenzyme A was deacylated by the enzyme, with a Km of 0.012 mM. S-Adenosylmethionine and S-adenosylhomocysteine slightly inhibited the enzyme, but methionine had no effect.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brush A., Paulus H. The enzymic formation of O-acetylhomoserine in Bacillus subtilis and its regulation by methionine and S-adenosylmethionine. Biochem Biophys Res Commun. 1971 Nov 5;45(3):735–741. doi: 10.1016/0006-291x(71)90478-5. [DOI] [PubMed] [Google Scholar]
- Cherest H., Eichler F., Robichon-Szulmajster H. Genetic and regulatory aspects of methionine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1969 Jan;97(1):328–336. doi: 10.1128/jb.97.1.328-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Döbeli H., Nüesch J. Regulatory properties of O-acetyl-L-serine sulfhydrylase of Cephalosporium acremonium: evidence of an isoenzyme and its importance in cephalosporin C biosynthesis. Antimicrob Agents Chemother. 1980 Jul;18(1):111–117. doi: 10.1128/aac.18.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Kerr D. S., Flavin M. The regulation of methionine synthesis and the nature of cystathionine gamma-synthase in Neurospora. J Biol Chem. 1970 Apr 10;245(7):1842–1855. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee L. W., Ravel J. M., Shive W. Multimetabolite control of a biosynthetic pathway by sequential metabolites. J Biol Chem. 1966 Nov 25;241(22):5479–5480. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Miyajima R., Shiio I. Regulation of aspartate family amino acid biosynthesis in Brevibacterium flavum. VII. Properities of homoserine O-transacetylase. J Biochem. 1973 May;73(5):1061–1068. doi: 10.1093/oxfordjournals.jbchem.a130160. [DOI] [PubMed] [Google Scholar]
- Morzycka E., Paszewski A. Cysteine and homocysteine synthesis in Saccharomycopsis lipolytica; identification and characterization of two cysteine synthases. Acta Biochim Pol. 1982;29(1-2):81–93. [PubMed] [Google Scholar]
- Ozaki H., Shiio I. Methionine biosynthesis in Brevibacterium flavum: properties and essential role of O-acetylhomoserine sulfhydrylase. J Biochem. 1982 Apr;91(4):1163–1171. doi: 10.1093/oxfordjournals.jbchem.a133799. [DOI] [PubMed] [Google Scholar]
- Paszewski A., Grabski J. On sulfhydrylation of O-acetylserine and O-acetylhomoserine in homocysteine synthesis in yeast. Acta Biochim Pol. 1976;23(4):321–324. [PubMed] [Google Scholar]
- Robichon-Szulmajster H., Cherest H. Regulation of homoserine O-transacetylase, first step in methionine biosyntheis in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1967 Jul 21;28(2):256–262. doi: 10.1016/0006-291x(67)90438-x. [DOI] [PubMed] [Google Scholar]
- Shiio I., Ozaki H. Feedback inhibition by methionine and S-adenosylmethionine, and desensitization of homoserine O-acetyltransferase in Brevibacterium flavum. J Biochem. 1981 May;89(5):1493–1500. doi: 10.1093/oxfordjournals.jbchem.a133342. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Wyman A., Paulus H. Purification and properties of homoserine transacetylase from Bacillus polymyxa. J Biol Chem. 1975 May 25;250(10):3897–3903. [PubMed] [Google Scholar]
- Wyman A., Shelton E., Paulus H. Regulation of homoserine transacetylase in whole cells of Bacillus polymyxa. J Biol Chem. 1975 May 25;250(10):3904–3908. [PubMed] [Google Scholar]
- Yamagata S. O-Acetylhomoserine sulfhydrylase of the fission yeast Schizosaccharomyces pombe: partial purification, characterization, and its probable role in homocysteine biosynthesis. J Biochem. 1984 Nov;96(5):1511–1523. doi: 10.1093/oxfordjournals.jbchem.a134980. [DOI] [PubMed] [Google Scholar]
- Yamagata S., Takeshima K., Naiki N. Evidence for the identity of O-acetylserine sulfhydrylase with O-acetylhomoserine sulfhydrylase in yeast. J Biochem. 1974 Jun;75(6):1221–1229. doi: 10.1093/oxfordjournals.jbchem.a130505. [DOI] [PubMed] [Google Scholar]
- Yamagata S., Takeshima K., Naiki N. O-acetylserine and O-acetylhomoserine sulfhydrylase of yeast; studies with methionine auxotrophs. J Biochem. 1975 May;77(5):1029–1036. doi: 10.1093/oxfordjournals.jbchem.a130803. [DOI] [PubMed] [Google Scholar]
- Yamagata S., Takeshima K. O-Acetylserine and O-acetylhomoserine sulfhydrylase of yeast. Further purification and characterization as a pyridoxal enzyme. J Biochem. 1976 Oct;80(4):777–785. doi: 10.1093/oxfordjournals.jbchem.a131338. [DOI] [PubMed] [Google Scholar]



