Abstract
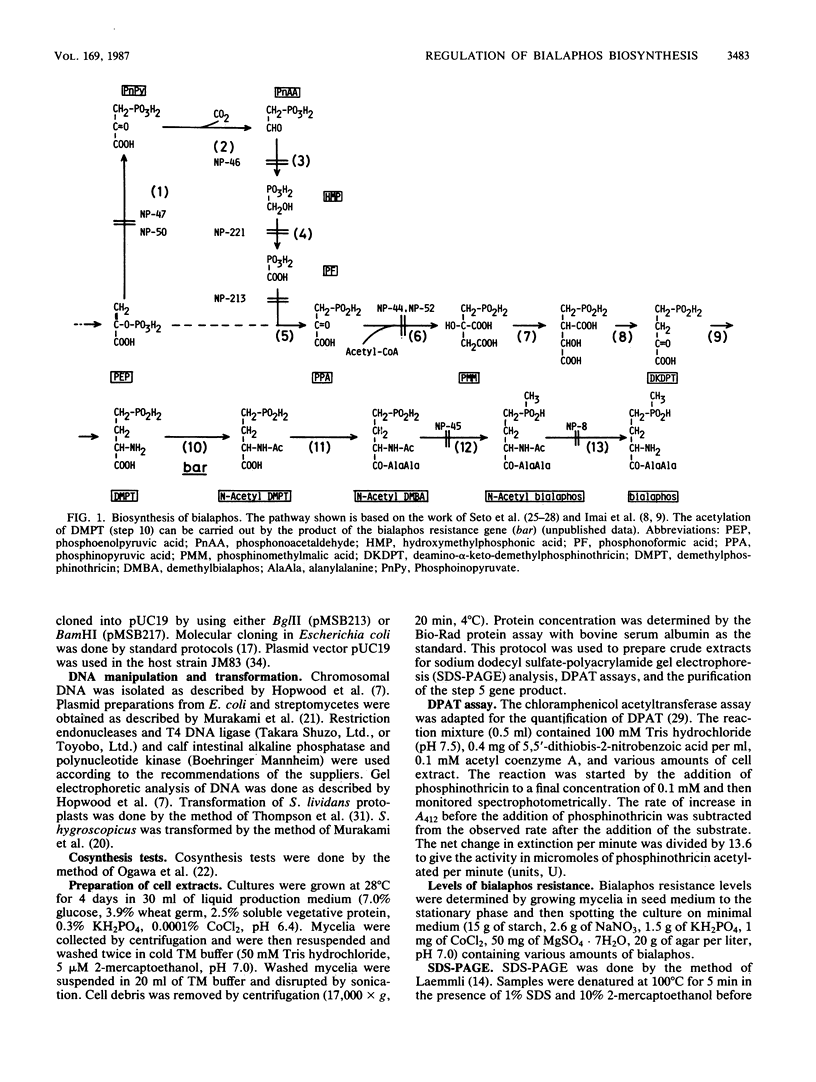
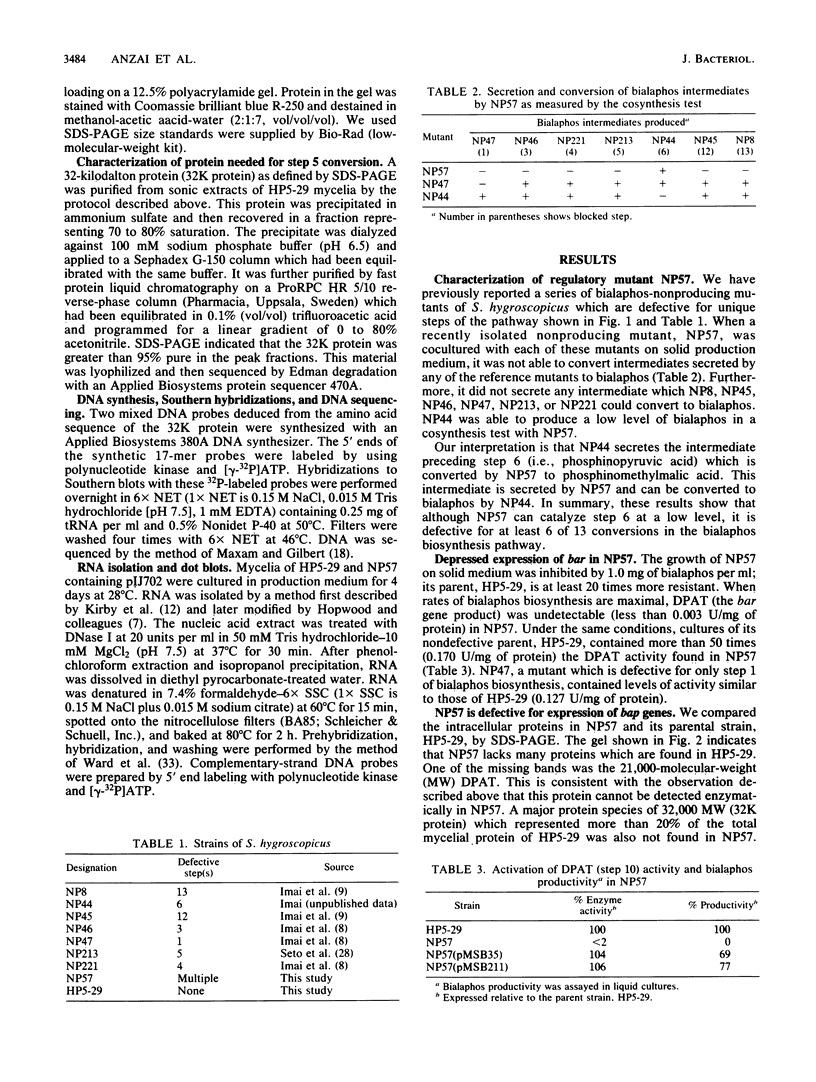
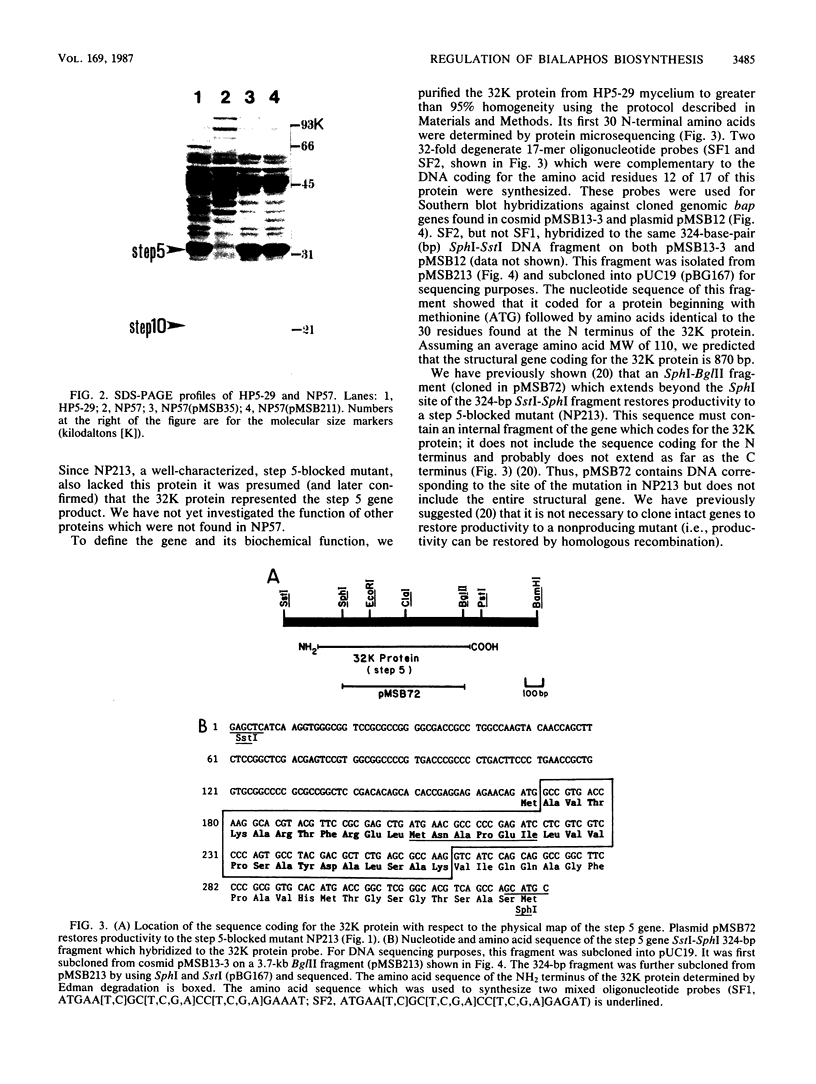
A DNA sequence (brpA) which regulates the expression of the genes of the bialaphos biosynthesis pathway (bap) in Streptomyces hygroscopicus was identified and characterized. A newly isolated nonproducing mutant (NP57) had a pleiotropic defect involving at least 6 of the 13 known bap genes; only the step 6 conversion could be detected. NP57 was more sensitive to bialaphos than its parent and had depressed levels of the demethylphosphinothricin acetyltransferase activity (step 10 in the pathway) which confers bialaphos resistance. Sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis of extracts of this mutant showed that it lacked proteins corresponding to steps 5 and 10. NP57 lacked mRNAs for steps 5, 10, and 13. Bialaphos productivity of NP57 was restored by transformation with a plasmid containing a 5.9-kilobase DNA fragment which was adjacent to the structural gene cluster. Subcloning experiments showed that a 1.3-kilobase fragment from this primary clone restored all the defects of NP57. We conclude that brpA can activate the transcription of the bialaphos resistance gene as well as at least six other bap structural genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E., Gugel K. H., Hägele K., Hagenmaier H., Jessipow S., König W. A., Zähner H. Stoffwechselprodukte von Mikroorganismen. 98. Phosphinothricin und Phosphinothricyl-Alanyl-Alanin. Helv Chim Acta. 1972 Jan 31;55(1):224–239. doi: 10.1002/hlca.19720550126. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Bruton C. J. Mutational cloning in Streptomyces and the isolation of antibiotic production genes. Gene. 1983 Dec;26(1):67–78. doi: 10.1016/0378-1119(83)90037-9. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Bruton C. J. Resistance, regulatory and production genes for the antibiotic methylenomycin are clustered. EMBO J. 1985 Jul;4(7):1893–1897. doi: 10.1002/j.1460-2075.1985.tb03866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitelson J. S., Hopwood D. A. Cloning of a Streptomyces gene for an O-methyltransferase involved in antibiotic biosynthesis. Mol Gen Genet. 1983;190(3):394–398. doi: 10.1007/BF00331065. [DOI] [PubMed] [Google Scholar]
- Hara O., Beppu T. Mutants blocked in streptomycin production in Streptomyces griseus - the role of A-factor. J Antibiot (Tokyo) 1982 Mar;35(3):349–358. doi: 10.7164/antibiotics.35.349. [DOI] [PubMed] [Google Scholar]
- Imai S., Seto H., Sasaki T., Tsuruoka T., Ogawa H., Satoh A., Inouye S., Niida T., Otake N. Studies on the biosynthesis of bialaphos (SF-1293). 4. Production of phosphonic acid derivatives, 2-hydroxyethylphosphonic acid, hydroxymethylphosphonic acid and phosphonoformic acid by blocked mutants of Streptomyces hygroscopicus SF-1293 and their roles in the biosynthesis of bialaphos. J Antibiot (Tokyo) 1984 Nov;37(11):1505–1508. doi: 10.7164/antibiotics.37.1505. [DOI] [PubMed] [Google Scholar]
- Imai S., Seto H., Sasaki T., Tsuruoka T., Ogawa H., Satoh A., Inouye S., Niida T., Otake N. Studies on the biosynthesis of bialaphos (SF-1293). 6. Production of N-acetyl-demethylphosphinothricin and N-acetylbialaphos by blocked mutants of Streptomyces hygroscopicus SF-1293 and their roles in the biosynthesis of bialaphos. J Antibiot (Tokyo) 1985 May;38(5):687–690. doi: 10.7164/antibiotics.38.687. [DOI] [PubMed] [Google Scholar]
- Katz E., Thompson C. J., Hopwood D. A. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol. 1983 Sep;129(9):2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- Khokhlov A. S., Anisova L. N., Tovarova I. I., Kleiner E. M., Kovalenko I. V., Krasilnikova O. I., Kornitskaya E. Y., Pliner S. A. Effect of A-factor on the growth of asporogenous mutants of Streptomyces griseus, not producing this factor. Z Allg Mikrobiol. 1973;13(8):647–655. doi: 10.1002/jobm.3630130803. [DOI] [PubMed] [Google Scholar]
- Kirby K. S., Fox-Carter E., Guest M. Isolation of deoxyribonucleic acid and ribosomal ribonucleic acid from bacteria. Biochem J. 1967 Jul;104(1):258–262. doi: 10.1042/bj1040258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. 1984 May 31-Jun 6Nature. 309(5967):462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Physical and genetic characterisation of the gene cluster for the antibiotic actinorhodin in Streptomyces coelicolor A3(2). Mol Gen Genet. 1986 Oct;205(1):66–73. doi: 10.1007/BF02428033. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Merrick M. J. A morphological and genetic mapping study of bald colony mutants of Streptomyces coelicolor. J Gen Microbiol. 1976 Oct;96(2):299–315. doi: 10.1099/00221287-96-2-299. [DOI] [PubMed] [Google Scholar]
- Murakami T., Nojiri C., Toyama H., Hayashi E., Yamada Y., Nagaoka K. Pock forming plasmids from antibiotic-producing Streptomyces. J Antibiot (Tokyo) 1983 Apr;36(4):429–434. doi: 10.7164/antibiotics.36.429. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Imai S., Shimizu T., Satoh A., Kojima M. Cosynthesis and protoplast fusion by mutants of bialaphos (AMPBA) producing Streptomyces hygroscopicus. J Antibiot (Tokyo) 1983 Aug;36(8):1040–1044. doi: 10.7164/antibiotics.36.1040. [DOI] [PubMed] [Google Scholar]
- Ohnuki T., Imanaka T., Aiba S. Self-cloning in Streptomyces griseus of an str gene cluster for streptomycin biosynthesis and streptomycin resistance. J Bacteriol. 1985 Oct;164(1):85–94. doi: 10.1128/jb.164.1.85-94.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queener S. W., Sebek O. K., Vézina C. Mutants blocked in antibiotic synthesis. Annu Rev Microbiol. 1978;32:593–636. doi: 10.1146/annurev.mi.32.100178.003113. [DOI] [PubMed] [Google Scholar]
- Seto H., Imai S., Sasaki T., Shimotohno K., Tsuruoka T., Ogawa H., Satoh A., Inouye S., Niida T., Otake N. Studies on the biosynthesis of bialaphos (SF-1293). 5. Production of 2-phosphinomethylmalic acid, an analogue of citric acid by Streptomyces hygroscopicus SF-1293 and its involvement in the biosynthesis of bialaphos. J Antibiot (Tokyo) 1984 Nov;37(11):1509–1511. doi: 10.7164/antibiotics.37.1509. [DOI] [PubMed] [Google Scholar]
- Seto H., Imai S., Tsuruoka T., Ogawa H., Satoh A., Sasaki T., Otake N. Studies on the biosynthesis of bialaphos (SF-1293) Part 3. Production of phosphinic acid derivatives, MP-103, MP-104 and MP-105, by a blocked mutant of Streptomyces hygroscopicus SF-1293 and their roles in the biosynthesis of bialaphos. Biochem Biophys Res Commun. 1983 Mar 29;111(3):1008–1014. doi: 10.1016/0006-291x(83)91400-6. [DOI] [PubMed] [Google Scholar]
- Seto H., Imai S., Tsuruoka T., Satoh A., Kojima M., Inouye S., Sasaki T., Otake N. Studies on the biosynthesis of bialaphos (SF-1293). 1. Incorporation of 13C- and 2H-labeled precursors into bialaphos. J Antibiot (Tokyo) 1982 Dec;35(12):1719–1721. doi: 10.7164/antibiotics.35.1719. [DOI] [PubMed] [Google Scholar]
- Seto H., Sasaki T., Imai S., Tsuruoka T., Ogawa H., Satoh A., Inouye S., Niida T., Otake N. Studies on the biosynthesis of bialaphos (SF-1293). 2. Isolation of the first natural products with a C-P-H bond and their involvement in the C-P-C bond formation. J Antibiot (Tokyo) 1983 Jan;36(1):96–98. doi: 10.7164/antibiotics.36.96. [DOI] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. Cloning of antibiotic resistance and nutritional genes in streptomycetes. J Bacteriol. 1982 Aug;151(2):668–677. doi: 10.1128/jb.151.2.668-677.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Janssen G. R., Kieser T., Bibb M. J., Buttner M. J., Bibb M. J. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol Gen Genet. 1986 Jun;203(3):468–478. doi: 10.1007/BF00422072. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]




