Abstract
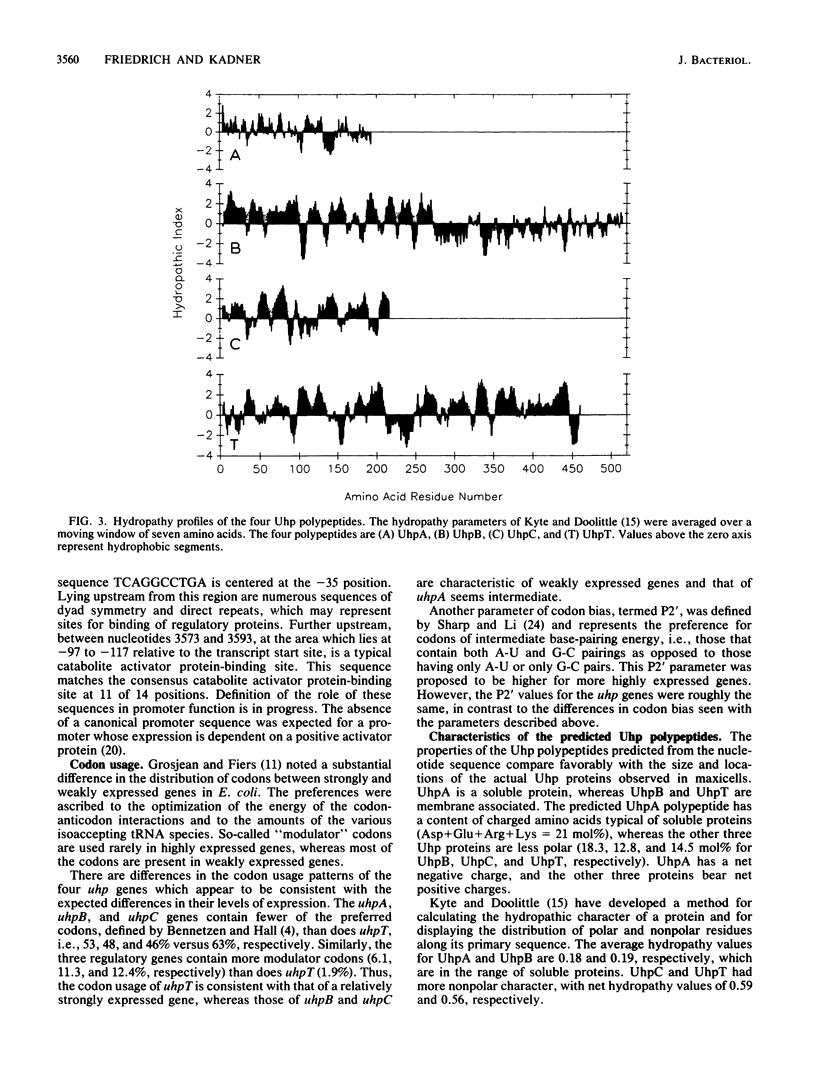
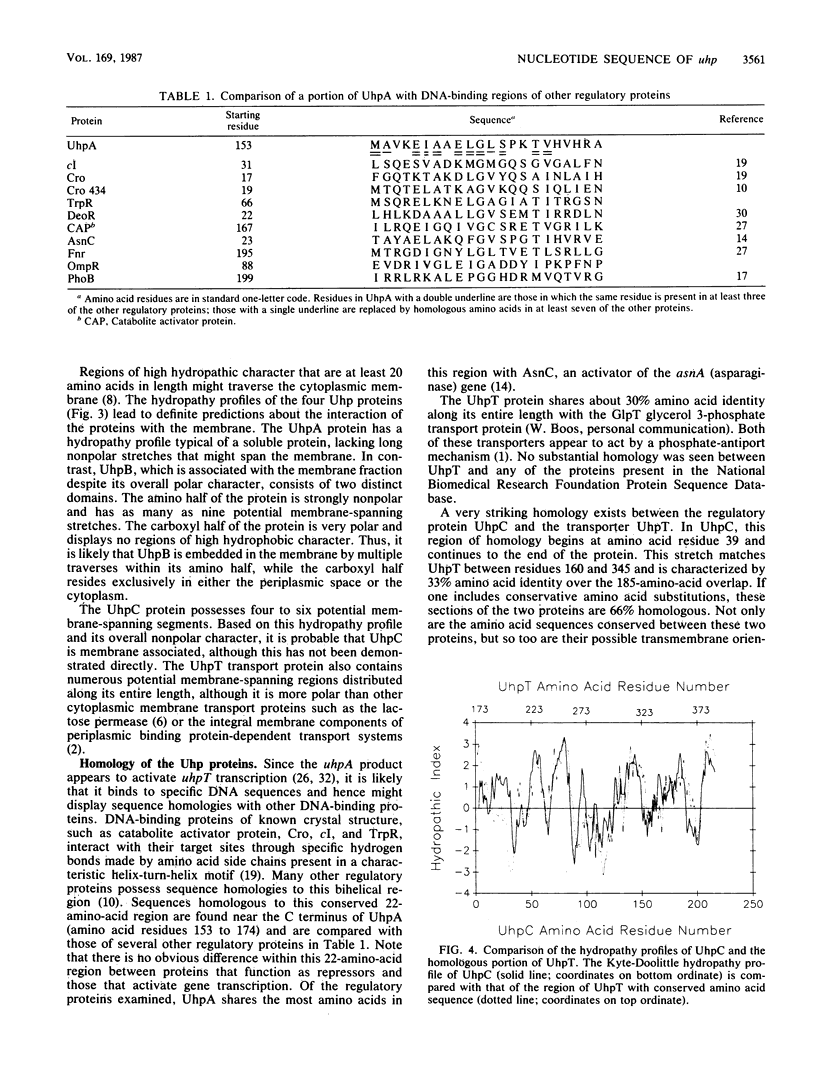
The Escherichia coli uhp region encodes the transport system that mediates the uptake of a number of sugar phosphates as well as the regulatory components that are responsible for induction of this transport system by external glucose 6-phosphate. Four uhp genes have been identified by analysis of the complementation behavior and polypeptide coding capacity of plasmids carrying subcloned regions or transposon insertions. The nucleotide sequence of a 6.5-kilobase segment that contains the 3' end of the ilvBN operon and the entire uhp region was determined. Four open reading frames were identified in the locations expected for the various uhp genes; all were oriented in the same direction, counterclockwise relative to the genetic map. The properties of the polypeptides predicted from the nucleotide sequence were consistent with their observed features. The 196-amino-acid UhpA polypeptide has the composition characteristic of a soluble protein and bears homology to the DNA-binding regions of many regulatory activators and repressors. The 518-amino-acid UhpB and the 199-amino-acid UhpC regulatory proteins contain substantial segments of hydrophobic character. Similarly, the 463-amino-acid UhpT transporter is a hydrophobic protein with numerous potential transmembrane segments. The UhpC regulatory protein has substantial sequence homology to part of UhpT, suggesting that this regulatory protein might have evolved by duplication of the gene for the transporter and that its role in transmembrane signaling may involve sugar-phosphate-binding sites and transmembrane orientations similar to those of the transport protein.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambudkar S. V., Larson T. J., Maloney P. C. Reconstitution of sugar phosphate transport systems of Escherichia coli. J Biol Chem. 1986 Jul 15;261(20):9083–9086. [PubMed] [Google Scholar]
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- Friden P., Donegan J., Mullen J., Tsui P., Freundlich M., Eoyang L., Weber R., Silverman P. M. The ilvB locus of Escherichia coli K-12 is an operon encoding both subunits of acetohydroxyacid synthase I. Nucleic Acids Res. 1985 Jun 11;13(11):3979–3993. doi: 10.1093/nar/13.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gicquel-Sanzey B., Cossart P. Homologies between different procaryotic DNA-binding regulatory proteins and between their sites of action. EMBO J. 1982;1(5):591–595. doi: 10.1002/j.1460-2075.1982.tb01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Kadner R. J. Genetic Control of the Transport of Hexose Phosphates in Escherichia coli: Mapping of the uhp Locus. J Bacteriol. 1973 Nov;116(2):764–770. doi: 10.1128/jb.116.2.764-770.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Shattuck-Eidens D. M. Genetic control of the hexose phosphate transport system of Escherichia coli: mapping of deletion and insertion mutations in the uhp region. J Bacteriol. 1983 Sep;155(3):1052–1061. doi: 10.1128/jb.155.3.1052-1061.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Kölling R., Lother H. AsnC: an autogenously regulated activator of asparagine synthetase A transcription in Escherichia coli. J Bacteriol. 1985 Oct;164(1):310–315. doi: 10.1128/jb.164.1.310-315.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Amemura M., Nakata A. Nucleotide sequence of the phoB gene, the positive regulatory gene for the phosphate regulon of Escherichia coli K-12. J Mol Biol. 1986 Jul 5;190(1):37–44. doi: 10.1016/0022-2836(86)90073-2. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Salser W. Globin mRNA sequences: analysis of base pairing and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):985–1002. doi: 10.1101/sqb.1978.042.01.099. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Li W. H. Codon usage in regulatory genes in Escherichia coli does not reflect selection for 'rare' codons. Nucleic Acids Res. 1986 Oct 10;14(19):7737–7749. doi: 10.1093/nar/14.19.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck-Eidens D. M., Kadner R. J. Exogenous induction of the Escherichia coli hexose phosphate transport system defined by uhp-lac operon fusions. J Bacteriol. 1981 Oct;148(1):203–209. doi: 10.1128/jb.148.1.203-209.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck-Eidens D. M., Kadner R. J. Molecular cloning of the uhp region and evidence for a positive activator for expression of the hexose phosphate transport system of Escherichia coli. J Bacteriol. 1983 Sep;155(3):1062–1070. doi: 10.1128/jb.155.3.1062-1070.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D. J., Rice D. W., Guest J. R. Homology between CAP and Fnr, a regulator of anaerobic respiration in Escherichia coli. J Mol Biol. 1983 May 15;166(2):241–247. doi: 10.1016/s0022-2836(83)80011-4. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Højrup P., Short S. The primary structure of the DeoR repressor from Escherichia coli K-12. Nucleic Acids Res. 1985 Aug 26;13(16):5927–5936. doi: 10.1093/nar/13.16.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Hauser C. A., Hatfield G. W. The nucleotide sequence of the ilvBN operon of Escherichia coli: sequence homologies of the acetohydroxy acid synthase isozymes. Nucleic Acids Res. 1985 Jun 11;13(11):3995–4010. doi: 10.1093/nar/13.11.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston L. A., Kadner R. J. Identification of uhp polypeptides and evidence for their role in exogenous induction of the sugar phosphate transport system of Escherichia coli K-12. J Bacteriol. 1987 Aug;169(8):3546–3555. doi: 10.1128/jb.169.8.3546-3555.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Compartmentation in the induction of the hexose-6-phosphate transport system of Escherichia coli. J Bacteriol. 1970 Feb;101(2):470–475. doi: 10.1128/jb.101.2.470-475.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



