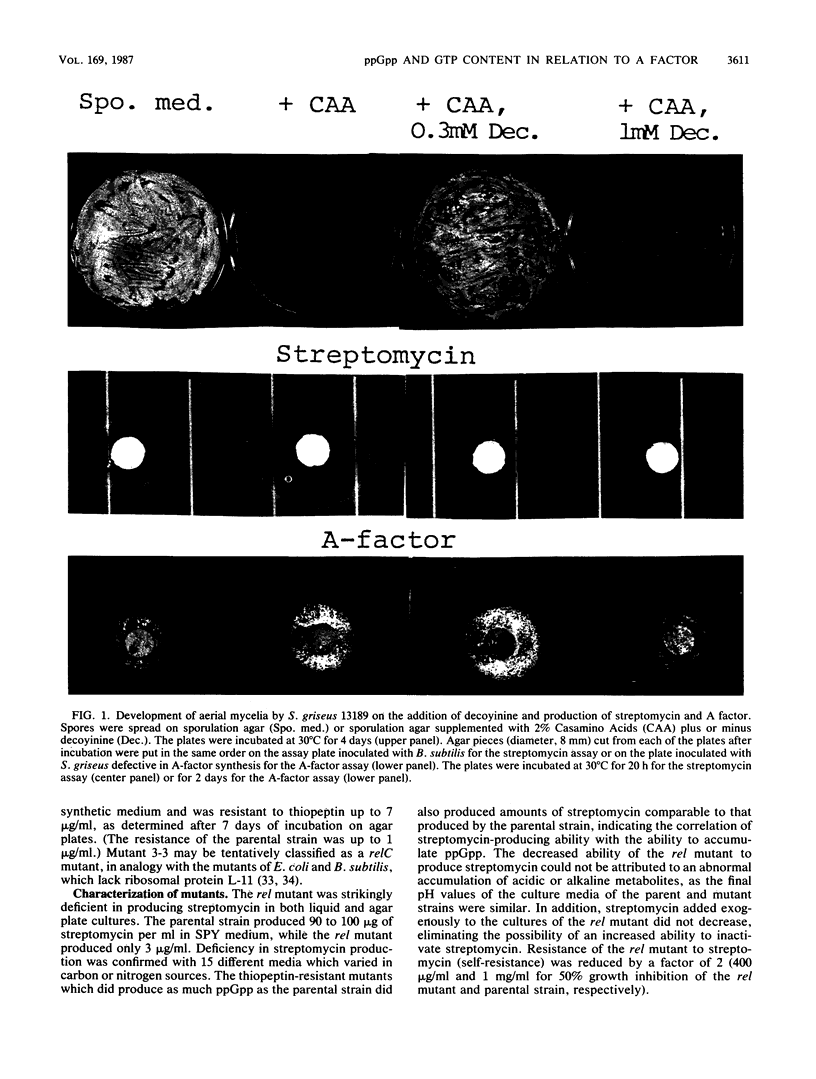
Abstract
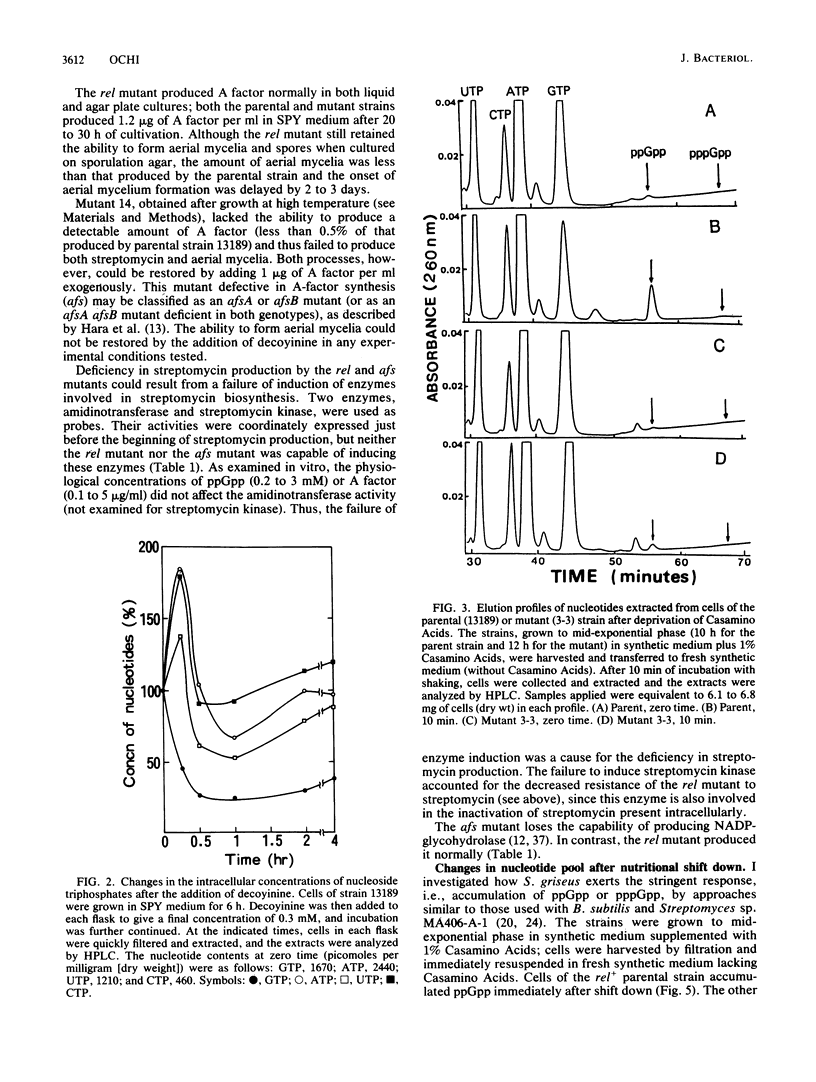
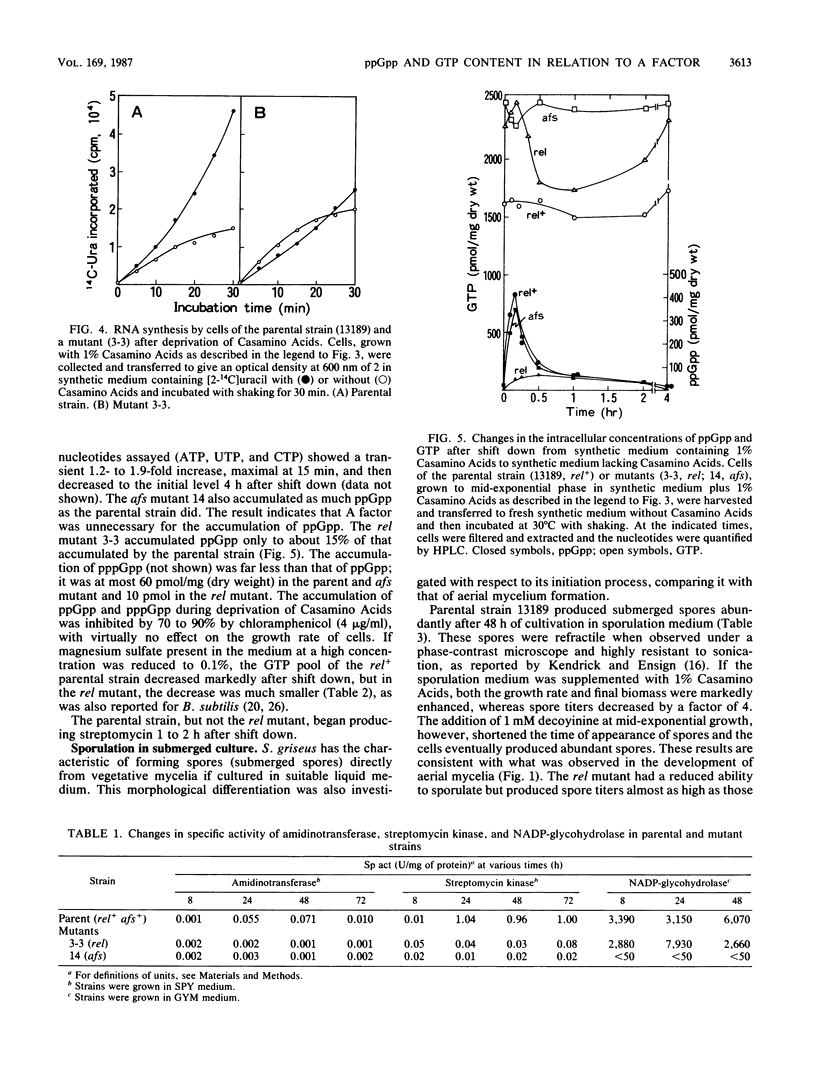
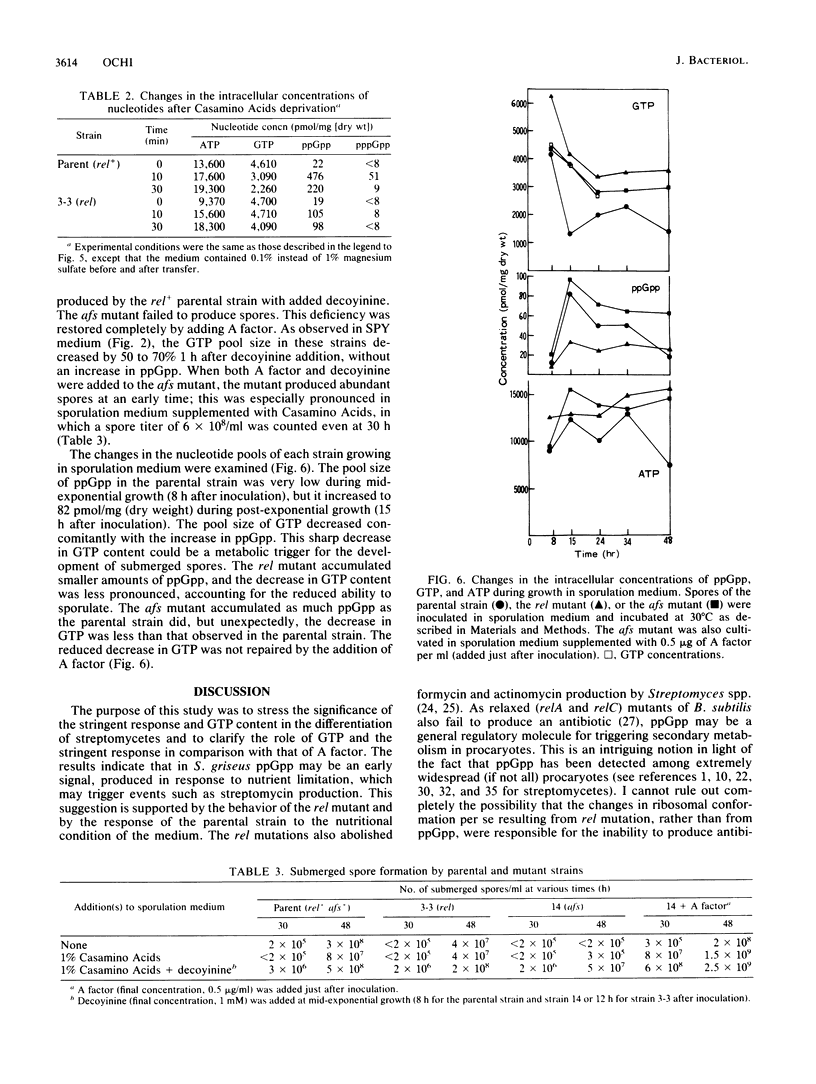
I investigated the significance of the intracellular accumulation of guanosine 5'-diphosphate 3'-diphosphate (ppGpp) and of the coordinated decrease in the GTP pool for initiating morphological and physiological differentiation of Streptomyces griseus, a streptomycin-producing strain. In solid cultures, aerial mycelium formation was severely suppressed by the presence of excess nutrients. However, decoyinine, a specific inhibitor of GMP synthetase, enabled the cells to develop aerial mycelia in the suppressed cultures at concentrations which only partially inhibited growth. A factor (2S-isocapryloyl-3S-hydroxymethyl-gamma-butyrolactone) added exogenously had no such effect. Decoyinine was also effective in initiating the formation of submerged spores in liquid culture. The ability to produce streptomycin did not increase but decreased drastically on the addition of decoyinine. This sharp decrease in streptomycin production was accompanied by a decrease in intracellular accumulation of ppGpp. A relaxed (rel) mutant was found among 25 thiopeptin-resistant isolates which developed spontaneously. The rel mutant had a severely reduced ability to accumulate ppGpp during a nutritional shift-down and also during postexponential growth and showed a less extensive decrease in the GTP pool than that in the rel+ parental strain. The rel mutant failed to induce the enzymes amidinotransferase and streptomycin kinase, which are essential for the biosynthesis of streptomycin. The abilities to form aerial mycelia and submerged spores were still retained, but the amounts were less, and for both the onset of development was markedly delayed. The decreased ability to produced submerged spores was largely restored by the addition of decoyinine. This was accompanied by an extensive GTP pool decrease. The rel mutant produced A factor normally, indicating that synthesis of A factor is controlled neither by ppGpp nor by GTP. Conversely, a mutant defective in A-factor synthesis accumulated as much ppGpp as did the parental strain. It was concluded that morphological differentiation of S. griseus results from a decrease in the pool of GTP, whereas physiological differentiation results from a more direct function of the rel gene product (ppGpp). It is also suggested that A factor may render the cell sensitive to receive and respond to the specified signal molecules, presumably ppGpp (for physiological differentiation) or GTP (for morphological differentiation).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Vining L. C. Intracellular levels of guanosine 5'-diphosphate 3'-diphosphate (ppGpp) and guanosine 5'-triphosphate 3'-diphosphate (pppGpp) in cultures of Streptomyces griseus producing streptomycin. Can J Microbiol. 1978 May;24(5):502–511. doi: 10.1139/m78-083. [DOI] [PubMed] [Google Scholar]
- Belitsky B., Kari C. Absence of accumulation of ppGpp and RNA during amino acid starvation in Rhizobium meliloti. J Biol Chem. 1982 May 10;257(9):4677–4679. [PubMed] [Google Scholar]
- Biró S., Békési I., Vitális S., Szabó G. A substance effecting differentiation in Streptomyces griseus. Purification and properties. Eur J Biochem. 1980 Jan;103(2):359–363. doi: 10.1111/j.1432-1033.1980.tb04322.x. [DOI] [PubMed] [Google Scholar]
- Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- Chiaverotti T. A., Parker G., Gallant J., Agabian N. Conditions that trigger guanosine tetraphosphate accumulation in Caulobacter crescentus. J Bacteriol. 1981 Mar;145(3):1463–1465. doi: 10.1128/jb.145.3.1463-1465.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Gallant J., Irr J., Cashel M. The mechanism of amino acid control of guanylate and adenylate biosynthesis. J Biol Chem. 1971 Sep 25;246(18):5812–5816. [PubMed] [Google Scholar]
- Gräfe U., Reinhardt G., Krebs D., Eritt I., Fleck W. F. Pleiotropic effects of a butyrolactone-type autoregulator on mutants of Streptomyces griseus blocked in cytodifferentiation. J Gen Microbiol. 1984 May;130(5):1237–1245. doi: 10.1099/00221287-130-5-1237. [DOI] [PubMed] [Google Scholar]
- Hara O., Beppu T. Induction of streptomycin-inactivating enzyme by A-factor in Streptomyces griseus. J Antibiot (Tokyo) 1982 Sep;35(9):1208–1215. doi: 10.7164/antibiotics.35.1208. [DOI] [PubMed] [Google Scholar]
- Hara O., Beppu T. Mutants blocked in streptomycin production in Streptomyces griseus - the role of A-factor. J Antibiot (Tokyo) 1982 Mar;35(3):349–358. doi: 10.7164/antibiotics.35.349. [DOI] [PubMed] [Google Scholar]
- Hara O., Horinouchi S., Uozumi T., Beppu T. Genetic analysis of A-factor synthesis in Streptomyces coelicolor A3(2) and Streptomyces griseus. J Gen Microbiol. 1983 Sep;129(9):2939–2944. doi: 10.1099/00221287-129-9-2939. [DOI] [PubMed] [Google Scholar]
- Hensens O. D., Albers-Schönberg G. Total structure of the highly modified peptide antibiotic components of thiopeptin. J Antibiot (Tokyo) 1983 Jul;36(7):814–831. doi: 10.7164/antibiotics.36.814. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Hara O., Beppu T. Cloning of a pleiotropic gene that positively controls biosynthesis of A-factor, actinorhodin, and prodigiosin in Streptomyces coelicolor A3(2) and Streptomyces lividans. J Bacteriol. 1983 Sep;155(3):1238–1248. doi: 10.1128/jb.155.3.1238-1248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick K. E., Ensign J. C. Sporulation of Streptomyces griseus in submerged culture. J Bacteriol. 1983 Jul;155(1):357–366. doi: 10.1128/jb.155.1.357-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlov A. S., Anisova L. N., Tovarova I. I., Kleiner E. M., Kovalenko I. V., Krasilnikova O. I., Kornitskaya E. Y., Pliner S. A. Effect of A-factor on the growth of asporogenous mutants of Streptomyces griseus, not producing this factor. Z Allg Mikrobiol. 1973;13(8):647–655. doi: 10.1002/jobm.3630130803. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lopez J. M., Dromerick A., Freese E. Response of guanosine 5'-triphosphate concentration to nutritional changes and its significance for Bacillus subtilis sporulation. J Bacteriol. 1981 May;146(2):605–613. doi: 10.1128/jb.146.2.605-613.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K. A decrease in GTP content is associated with aerial mycelium formation in Streptomyces MA406-A-1. J Gen Microbiol. 1986 Feb;132(2):299–305. doi: 10.1099/00221287-132-2-299. [DOI] [PubMed] [Google Scholar]
- Ochi K., Kandala J. C., Freese E. Initiation of Bacillus subtilis sporulation by the stringent response to partial amino acid deprivation. J Biol Chem. 1981 Jul 10;256(13):6866–6875. [PubMed] [Google Scholar]
- Ochi K. Occurrence of the stringent response in Streptomyces sp. and its significance for the initiation of morphological and physiological differentiation. J Gen Microbiol. 1986 Sep;132(9):2621–2631. doi: 10.1099/00221287-132-9-2621. [DOI] [PubMed] [Google Scholar]
- Ochi K., Ohsawa S. Initiation of antibiotic production by the stringent response of Bacillus subtilis Marburg. J Gen Microbiol. 1984 Oct;130(10):2473–2482. doi: 10.1099/00221287-130-10-2473. [DOI] [PubMed] [Google Scholar]
- Ochi K., Saito Y., Umehara K., Ueda I., Kohsaka M. Restoration of aerial mycelium and antibiotic production in a Streptomyces griseoflavus arginine auxotroph. J Gen Microbiol. 1984 Aug;130(8):2007–2013. doi: 10.1099/00221287-130-8-2007. [DOI] [PubMed] [Google Scholar]
- Riesenberg D., Bergter F., Kari C. Effect of serine hydroxamate and methyl alpha-D-glucopyranoside treatment on nucleoside polyphosphate pools, RNA and protein accumulation in Streptomyces hygroscopicus. J Gen Microbiol. 1984 Oct;130(10):2549–2558. doi: 10.1099/00221287-130-10-2549. [DOI] [PubMed] [Google Scholar]
- Simúth J., Hudec J., Chau H. T., Dányi O., Zelinka J. The synthesis of highly phosphorylated nucleotides, RNA and protein by Streptomyces aureofaciens. J Antibiot (Tokyo) 1979 Jan;32(1):53–58. doi: 10.7164/antibiotics.32.53. [DOI] [PubMed] [Google Scholar]
- Smith I., Paress P., Cabane K., Dubnau E. Genetics and physiology of the rel system of Bacillus subtilis. Mol Gen Genet. 1980;178(2):271–279. doi: 10.1007/BF00270472. [DOI] [PubMed] [Google Scholar]
- Smith I., Paress P., Pestka S. Thiostrepton-resistant mutants exhibit relaxed synthesis of RNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5993–5997. doi: 10.1073/pnas.75.12.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. B. Pathways of biosynthesis of the guanidinated inositol moieties of streptomycin and bluensomycin. Methods Enzymol. 1975;43:429–433. doi: 10.1016/0076-6879(75)43097-x. [DOI] [PubMed] [Google Scholar]