Abstract
Butterflies are among nature's most colourful animals, and provide a living showcase for how extremely bright, chromatic and iridescent coloration can be generated by complex optical mechanisms. The gross characteristics of male butterfly colour patterns are understood to function for species and/or sex recognition, but it is not known whether female mate choice promotes visual exaggeration of this coloration. Here I show that females of the sexually dichromatic species Hypolimnas bolina prefer conspecific males that possess bright iridescent blue/ultraviolet dorsal ornamentation. In separate field and enclosure experiments, using both dramatic and graded wing colour manipulations, I demonstrate that a moderate qualitative reduction in signal brightness and chromaticity has the same consequences as removing the signal entirely. These findings validate a long-held hypothesis, and argue for the importance of intra- versus interspecific selection as the driving force behind the exaggeration of bright, iridescent butterfly colour patterns.
Keywords: female mate choice, Hypolimnas, Lepidoptera, ornamentation, sexual selection, ultraviolet
1. Introduction
The stunning diversity of butterfly wing coloration has long offered a fertile ground for the study of genetics and evolution. From the work of Darwin (1874) and Wallace (1889), researchers have used butterfly colour patterns to inform and develop theories relating to crypsis (Cott 1940), mimicry (Bates 1862), speciation (Silberglied & Taylor 1978), genetic polymorphism (Ford 1964) and, more recently, evolutionary and developmental plasticity (Beldade et al. 2002). In recent times, butterflies have also emerged as a key system for understanding the mechanisms of colour production. While many colours result primarily from pigments, the brightest, most chromatic and iridescent coloration is generated by arrays of surface nanostructures, such as two-dimensional photonic crystals (Vukusic & Hooper 2005). Butterflies display an unrivalled range and complexity of structural colours and colour-producing mechanisms (Vukusic & Sambles 2003), and this diversity continues to inspire considerable biological and optical inquiry (e.g. Vukusic & Hooper 2005; Prum et al. 2006).
Much research has been motivated by the brightness and chromaticity of butterfly wing coloration; however, relative to other colourful animal groups (e.g. birds, fishes and amphibians), little is known about the selective factors that promote the most extreme visual exaggeration in this group. Since male butterflies are often considerably more conspicuous than conspecific females (at least in palatable, non-mimic species), many—beginning with Darwin (1874)—have speculated that the brightest wing coloration results from female mating preferences for exaggerated visual signals (although see Silberglied 1984). This view is supported by the observation that bright colours are frequently limited to the male's dorsal wing surfaces, which are exposed during flight, and contrast highly with the dull or ‘protective’ ventral patterning typically displayed while at rest. Further evidence is given by species in which the females occur as putative mimics of known distasteful species, while the males have retained the conspicuous, non-mimetic colour patterns ancestral to the group (e.g. Hypolimnas misippus; Stride 1958). In some of these cases, the females, but not the males, are polymorphic for colour pattern, and/or exhibit more pronounced phenotypic plasticity (Kemp & Jones 2001), which implies the presence of a more strongly stabilizing selection on male coloration. These factors are all consistent with Darwin's (1874) female preference-based hypothesis. However, there are other ways in which sexual and/or natural selection could generate such patterns (Turner 1978), and empirical data demonstrating female preferences for exaggerated male coloration are ultimately required.
There are a number of behavioural studies that demonstrate colour-based mate choice in butterflies (e.g. Stride 1958; Silberglied & Taylor 1978; Fordyce et al. 2002; Ellers & Boggs 2003; Sweeney et al. 2003; Robertson & Monteiro 2005). These data support the idea that exaggerated male (and sometimes female) coloration results from mate preferences, but their utility in this context is subject to one or several limitations. First and foremost, almost all studies have employed gross manipulations of colour pattern, such as the complete removal of one or a combination of discrete colour patches (e.g. Silberglied & Taylor 1978). This approach may demonstrate a female mating preference, but it is unclear whether the preference is based upon the need for mate recognition, or whether it represents a ‘true’ preference for visual signal exaggeration (as Darwin 1874 proposed). No study has yet used graded, quantified manipulations of existing colour patterns to show that female butterflies prefer the males bearing the brightest wing markings, which are ultimately required for an evaluation of signal exaggeration. Secondly, several studies have manipulated small, relatively inconspicuous (e.g. Wiernasz 1989; Fordyce et al. 2002) or sexually monomorphic (e.g. Robertson & Monteiro 2005) colour pattern elements, rather than focusing on the most exaggerated and/or sexually dimorphic coloration. As noted, exaggerated butterfly wing coloration is notable for its mechanistic innovation (Vukusic & Sambles 2003), and there is value in trying to understand the selective pressures leading to such innovation. Finally, owing to the logistical difficulties with measuring female mate choice in this taxon (for an in-depth treatment, see Silberglied 1984), researchers have tended to focus on male, rather than female, mating preferences (e.g. Knüttel & Fiedler 2001; Fordyce et al. 2002; Ellers & Boggs 2003; Sweeney et al. 2003). Male mate choice is more likely to reinforce stronger existing vectors of viability selection rather than selecting for signal exaggeration (Bonduriansky 2001), and ultimately bears limited relevance to the question of why the males themselves often tend to be the more visually exaggerated sex in this group.
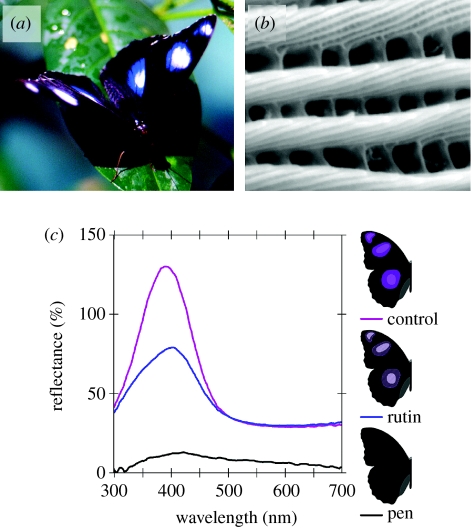
In this study, I set out to investigate the relevance of exaggerated male ornamentation to female mating decisions in the highly sexually dichromatic butterfly Hypolimnas bolina (Nymphalidae). Males, but not females, of this tropical species possess bright and conspicuous patches of bluish-UV (hereafter UV) iridescence resulting from dedicated surface multilayers on their dorsal wings (figure 1a,b; Kemp & Macedonia 2006). This UV occurs in ovoid-shaped patches that slightly overlap diffusely reflecting white spots; thus, each colour patch consists of a concentric ring of pure UV surrounding an inner UV+white (i.e. true insect white) spot. These patches are set against a matte black background, and are thus the defining feature of the male's dorsal coloration. Males present their dorsal wing surfaces to females in highly ritualized courtship manoeuvres, and available evidence suggests that this coloration does not have strong function during direct male–male (territorial) competition (Rutowski 1992; Kemp & Macedonia 2006). Here, I report the results of enclosure- and field-based experiments designed to test the hypothesis that females prefer males bearing the most visually exaggerated UV colour patches. I used both dramatic (i.e. complete removal of the UV reflectance) and graded (i.e. a reduction in signal brightness/chromaticity) wing colour manipulations, which allowed assessment of whether females are sensitive to the qualitative properties of the UV signal, and not merely the gross presence/absence of this conspicuous colour pattern element.
Figure 1.
(a) Male Hypolimnas bolina, showing the ovoid dorsal colour patches which consist of a diffusely reflecting white spot overlaid with iridescent bluish-UV. (b) Scanning electron micrograph (21 400×) showing the tilted multilayering on the surface of a wing scale responsible for the male's iridescent coloration. (c) Reflectance spectra taken from the centre of the larger colour patch on two different forewings that were untreated (control), then manipulated by painting with rutin, or blacking out with pen (refer to §2). Forewings were from the same individual and reflectance is relative to an MgO standard; an Ocean Optics USB-2000 spectrometer was used with a pulsed PX-2 xenon light source.
2. Material and methods
(a) Experimental enclosure
Flight cage experiments were conducted in a large outdoor insectary (dimensions: 6×15×4 m) located at James Cook University, Cairns, Australia (16°53′ S, 145°45′ E). The insectary was covered in 32% UV-absorbing woven shade cloth and fitted out with forest mulch and tropical palms to mimic a rainforest corridor environment. Hypolimnas respond well to captivity, and this enclosure has been used previously to study mating behaviour (Kemp 2001) and successfully house individual male H. bolina for up to 45 days (Kemp 2002).
(b) Rearing protocols
Adult butterflies were the offspring of six female H. bolina caught near Cairns on 3–4 January 2006. Larvae were reared on Asystasia gangetica under semi-controlled conditions of 28±2°C (day) and 23±2°C (night), and field photoperiod regimes for Cairns in January and February. Freshly emerged adults were marked on their ventral hindwing with a small identifying number in gold ink and stored individually within gauze-topped plastic cups at 18±0.5°C and 14 : 10 L : D photoperiod. Adults were given ad libitum access to a 15% solution of sugar water during their time in storage, which was in all cases less than 5 days.
(c) Experiment 1: insectary black ink manipulation
In the first experiment, conducted during 8–17 February 2006, treatment males had their dorsal wing coloration completely blacked out (in a 4°C room using a black Sharpie marker; see the reflectance spectrum for ‘pen’ in figure 1c) and control males had ink applied to an equivalent area of their black dorsal ‘ground’ colour. Fifty lab-reared males in each group were then initially liberated into the insectary, along with 50 virgin females; thus, N=150 butterflies. The cage was surveyed at 20 min intervals (during the period of butterfly activity, 08.00–16.00 h; Rutowski 1992; Kemp 2001) and each mating pair was removed and immediately substituted with a virgin female and a male from the appropriate experimental group. Copulation between virgins of this species under outdoor cage conditions lasts for 50–60 min (Kemp 2001). Males are territorial (Rutowski 1992; Kemp 2002), but this behaviour is subdued when many males are confined in a relatively small area (D. J. Kemp 2000, personal observation). Nevertheless, to confirm that males of each experimental group had equal access to females, I surveyed the cage hourly over 4 days during the experiment and recorded the provenance (treatment or control) of males engaging in solo courtships.
(d) Experiment 2: field black ink manipulation
In the second experiment, conducted during 18–28 February 2006, virgin females were presented sequentially to a series of free-flying territorial resident males around Cairns. The males had been previously captured, chilled, numbered and randomly designated for either treatment or control manipulations as per experiment 1, then released. Only males with fresh or slightly worn wings were used, and only one female was presented to each male (i.e. each male and female were used only once). Females were transported to the field sites in plastic cups placed within an ice-filled cooler, and allowed to warm up within their cup prior to release. I timed the duration of male–female interactions from the point at which the male approached to within 10 cm until the pair either copulated or the male broke off and returned to his perching site.
(e) Experiment 3: flight cage rutin manipulation
The third experiment, conducted during 15–23 March 2006, was procedurally identical to the first cage experiment except that wing manipulations were performed using a saturated solution of rutin in ethanol (rather than the black ink). The rutin solution was obtained by agitating 1 g of rutin (Life Extension Corp.) in 10 ml of ethanol at 60°C for 20 min, allowing this to stand for 1 h and then drawing off the top. When applied to the dorsal wing spots, this solution reduced the peak brightness of the iridescent UV reflectance by approximately 50%, as confirmed by reflectance spectrometry (see ‘rutin’ in figure 1c), and which falls within the naturally occurring range of UV brightness variation in this species (Kemp & Macedonia 2006).
3. Results
(a) Experiment 1: insectary black ink manipulation
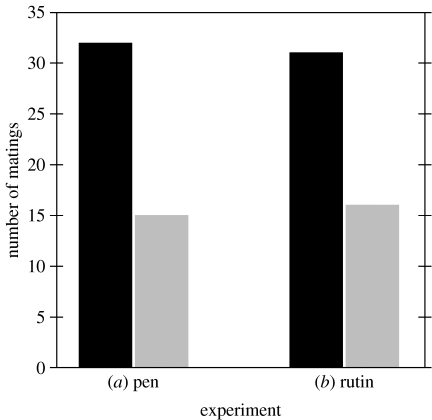
A total of 47 matings were observed during this experiment, with control males (32 matings (68%)) more likely to mate than their ‘blacked-out’ treatment counterparts (χ12=6.15, p<0.05; figure 2). Equal numbers of males from each group were seen engaging in solo courtships (116 control versus 122 treatment males; χ12=0.15, p=0.70), which suggests that both groups had equivalent access to females. The difference in mating success is therefore consistent with a female preference for males possessing the conspicuous dorsal colour patches.
Figure 2.
The number of copulations achieved by control males (black columns) versus treatment males (grey columns) in each of the two enclosure-based mate choice experiments (‘pen’ and ‘rutin’). The visual nature of each manipulation is shown in figure 1c.
(b) Experiment 2: field black ink manipulation
A total of 33 females were presented to prior manipulated males (control or treatment; as mentioned previously) at field territories. As with the enclosure-based experiment, control males (nine successful versus six unsuccessful) were more likely to mate than treatment males (4 successful versus 14 unsuccessful; χ12=4.99, p<0.05). In addition, among the males that achieved copulation, control males did so significantly faster (t11=3.97, p<0.005), which further suggests that females prefer males bearing this conspicuous dorsal coloration.
(c) Experiment 3: flight cage rutin manipulation
As with the first enclosure-based experiment, a total of 47 matings were observed in this experiment, and significantly more of these involved control males (31 control versus 16 treatment: χ12=4.79, p<0.05). The size of this effect did not differ from that of the first enclosure experiment (logistic regression: G1=0.05, p=0.83); thus indicating that this qualitative reduction in peak UV reflectance (figure 1c) had an equivalently negative effect on male mating success as did removal of the signal entirely (figure 2).
4. Discussion
Although butterflies are one of nature's most colourful animal groups, relatively little is known of the selective factors that promote their most visually exaggerated and mechanistically innovative coloration. In this study, I used the sexually dimorphic species H. bolina to address the hypothesis that qualitative ‘visual performance’ attributes of bright, male-limited coloration are selected in the context of female mate choice. The results express several key features. First, the entire removal of the male's conspicuous dorsal colour patches (the bluish-UV and inner white patches; figure 1a) significantly reduced the likelihood of successful copulation, and this effect was evident both under high-density cage conditions and with individual virgin female presentations in the wild. In the latter case, control males also required significantly shorter courtships than did those treatment males that ultimately managed to achieve copulation (a non-trivial number of blacked-out treatment males mated in both the field and the cage, perhaps indicating that non-visual cues also play a role in mate selection). The similarity of the enclosure- and field-based results, despite the drastically different ecological circumstances, strengthens the conclusion that this aspect of male coloration is important to male courtship success, and the most likely explanation is that females prefer males bearing this dorsal colour pattern element. Second, as shown by the third (rutin manipulation) experiment, a similar reduction in male courtship success can be elicited by a qualitative reduction in the brightness and chromaticity of only the iridescent UV. Analogy with the first two experiments suggests that this effect also derives from female mate choice, and, henceforth, that females not only prefer males bearing the iridescent UV patches, but also prefer relatively bright and chromatic UV reflectance. The importance of this finding lies in its potential to explain why some male butterflies display extreme levels of costly (Lyytinen et al. 2004) visual exaggeration, and perhaps also why they have invented a myriad mechanisms for achieving this brilliance (e.g. Vukusic & Sambles 2003; Vukusic & Hooper 2005; Prum et al. 2006).
The present results make a qualitative advance upon prior manipulative research into butterfly coloration in which only gross pattern aspects were altered (e.g. Silberglied & Taylor 1978; Fordyce et al. 2002; although see Robertson & Monteiro 2005, for the use of a rutin solution to manipulate male UV in Bicyclus). The finding that a reduction in the brilliance of the UV signal had the same consequences as its complete obliteration suggests that signal brightness is important, at least within the observed range of variation (figure 1c), and is consistent with female H. bolina possessing some acceptance threshold for this characteristic. Unfortunately, due to the technical difficulties with increasing the brightness of iridescent UV reflectance, it was not possible to determine the shape of the female preference function above the observed levels of signal brightness (i.e. to see whether females increasingly favour increasingly bright UV signals). Whether female mate choice would place directional or stabilizing selection on male UV brightness therefore remains to be seen. It would also be interesting to know whether graded variation in levels of brightness between the ‘control’ and the rutin males (as shown in figure 1c) would result in graded variation in female responses. Male bluish-UV certainly varies over this range in the wild, with the major source of variation attributable to age (i.e. wing wear; see Kemp & Macedonia 2006), and other butterflies have been shown to distinguish subtle variation in the hue and brightness of wing colours (e.g. Silberglied & Taylor (1978) and Ellers & Boggs (2003) regarding Colias spp.). Another interesting consideration is the extent to which bright UV markings would make male H. bolina more conspicuous—and, hence, more vulnerable—to visually orienting predators such as birds. Lyytinen et al. (2004) have recently shown that a 15% increase in wing UV reflectance rendered tethered moths significantly more likely to be preyed upon. Again, a 15% difference falls well within the range of naturally occurring variation in the peak of the bluish-UV in H. bolina (Kemp & Macedonia 2006). Costs due to increased predation are known to oppose the exaggeration of ornamental colour in other animals (e.g. guppies; Endler 1980), and appear at least to have the potential to do so in highly ornamented butterflies such as H. bolina.
Variation in the visual aspects (i.e. brightness, chroma and hue) of structural coloration may stem from variation in the density of the nanostructures across the surface, and/or variation in the anatomical precision with which the individual nanostructural elements are constructed (Shawkey et al. 2003; Kemp et al. 2006). Various workers have suggested, on this basis, that structurally coloured ornaments may provide reliable information regarding individual phenotypic and/or genetic quality, or age (Fitzpatrick 1998; Andersson 1999; Hill et al. 2005; Kemp & Rutowski 2007). Iridescent ornamentation has been linked to mate choice in birds (e.g. Hunt et al. 1999) and fishes (e.g. Kodric Brown & Nicoletto 1996), and has been shown to carry phenotypic quality information (Johnsen et al. 2003; Hill et al. 2005). In butterflies, iridescent wing markings have known function for (at least) mate recognition (Sweeney et al. 2003), but the present study provides the first experimental evidence that females may be sensitive to intraspecific variation in this trait. While the prime consequence of this behaviour would probably be selection for signal brightness (as mentioned previously), its evolutionary origin is more difficult to determine. Aside from the possibility of increased accuracy in mate recognition, it is unclear what other evolutionary benefits might accrue from choosing males with relatively bright UV. One potentially important consequence, given that UV brightness in male H. bolina declines predictably with age (Kemp & Macedonia 2006), is that females would tend to choose younger mates, which has been argued as a desirable outcome for female butterflies striving for a high-quality male ejaculate (Rutowski 1985). Furthermore, in Colias eurytheme, a species in which males exhibit UV iridescence arising from surface structures analogous to those of H. bolina—signal brightness has been shown as strongly heritable and phenotypically condition dependent (Kemp & Rutowski 2007). The latter finding implies that building the surface arrays may be nutritionally costly, and henceforth, that the expression of UV may signal an individual's phenotypic condition, and again, his ability to provide a nutritious ejaculate (Kemp & Rutowski 2007). These possibilities remain to be investigated in H. bolina.
Finally, the present experimental data complement and extend preliminary data obtained almost 50 years ago for the congeneric H. misippus, in which Stride (1958) presented virgin females to a series of variously manipulated males under flight cage conditions. As with H. bolina, male (but not female) H. misippus possess the distinctive dorsal colour scheme of ovoid white spots overlain with iridescent bluish-UV. In Stride's experiments, females were presented with: (i) ‘untreated’ males, (ii) ‘black’ males, who had their white spots, but not the bluish-UV, blacked out using a chlorazol solution, and (iii) ‘colourless’ males, who had most of their wing scales removed. Although the design was small and not amenable to statistics (N=9 males), Stride's data show very little difference in the courtship success of untreated and black males, but a clear tendency against successful courtship in colourless males. He concluded that the male's dorsal white spots have little function in courtship, but that important visual stimuli reside in the black areas of the wings, which (owing to the nature of his chlorazol manipulation) includes the iridescent patches. These results, although limited and lacking appropriate controls, are in agreement with those obtained here, and suggest that intraspecific female preferences of this nature may be commonplace at least among Hypolimnas, if not other colourful butterfly genera.
Acknowledgments
I thank R. Ennis-Thomas, M. Liddell, A. Monteiro and K.A. Robertson for their advice on using rutin to manipulate wing reflectance, and J. M. Macedonia for spectrometry and electron microscopy. J. Alcock, J. Merry, A. Monteiro, R. Rutowski and D. G. Stavenga provided helpful editorial suggestions. This work was supported by Australian Research Council grant no. DP0557190.
References
- Andersson S. Morphology of UV reflectance in a whistling-thrush: implications for the study of structural colour signalling in birds. J. Avian Biol. 1999;30:193–204. [Google Scholar]
- Bates H.W. Contributions to an insect fauna of the Amazon valley. Trans. Linn. Soc. Lond. 1862;23:495–566. [Google Scholar]
- Beldade P, Koops K, Brakefield P.M. Developmental constraints versus flexibility in morphological evolution. Nature. 2002;416:844–847. doi: 10.1038/416844a. doi:10.1038/416844a [DOI] [PubMed] [Google Scholar]
- Bonduriansky R. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. 2001;76:305–339. doi: 10.1017/s1464793101005693. doi:10.1017/S1464793101005693 [DOI] [PubMed] [Google Scholar]
- Cott H.B. Methuen & Co; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Darwin C. Murray; London, UK: 1874. The descent of man and selection in relation to sex. [Google Scholar]
- Ellers J, Boggs C.L. The evolution of wing color: male mate choice opposes adaptive wing color divergence in Colias butterflies. Evolution. 2003;57:1100–1106. doi: 10.1111/j.0014-3820.2003.tb00319.x. doi:10.1554/0014-3820(2003)057[1100:TEOWCM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Endler J.A. Natural selection on color patterns in Poecilia reticulata. Evolution. 1980;34:76–91. doi: 10.1111/j.1558-5646.1980.tb04790.x. doi:10.2307/2408316 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick S. Colour schemes for birds: structural coloration and signals of quality in feathers. Ann. Zool. Fenn. 1998;35:67–77. [Google Scholar]
- Ford E.B. Chapman & Hall; London, UK: 1964. Ecological genetics. [Google Scholar]
- Fordyce J.A, Nice C.C, Forister M.L, Shapiro A.M. The significance of wing pattern diversity in the Lycaenidae: mate discrimination by two recently diverged species. J. Evol. Biol. 2002;15:871–879. doi:10.1046/j.1420-9101.2002.00432.x [Google Scholar]
- Hill G.E, Doucet S.M, Buchholz R. The effect of coccidial infection on iridescent plumage coloration in wild turkeys. Anim. Behav. 2005;69:387–394. doi:10.1016/j.anbehav.2004.03.013 [Google Scholar]
- Hunt S, Cuthill I.C, Bennett A.T.D, Griffiths R. Preferences for ultraviolet partners in the blue tit. Anim. Behav. 1999;58:809–815. doi: 10.1006/anbe.1999.1214. doi:10.1006/anbe.1999.1214 [DOI] [PubMed] [Google Scholar]
- Johnsen A, Delhey K, Andersson S, Kempenaers B. Plumage colour in nesting blue tits: sexual dichromatism, condition dependence and genetic effects. Proc. R. Soc. B. 2003;270:1263–1270. doi: 10.1098/rspb.2003.2375. doi:10.1098/rspb.2003.2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D.J. The ecology of female receptivity in the territorial butterfly Hypolimnas bolina (L.) (Nymphalidae): implications for mate location by males. Aust. J. Zool. 2001;49:203–211. doi:10.1071/ZO01027 [Google Scholar]
- Kemp D.J. Sexual selection constrained by life history in a butterfly. Proc. R. Soc. B. 2002;269:1341–1346. doi: 10.1098/rspb.2002.2000. doi:10.1098/rspb.2002.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D.J, Jones R.E. Phenotypic plasticity in field populations of the tropical butterfly Hypolimnas bolina (L.) (Nymphalidae) Biol. J. Linn. Soc. 2001;72:33–45. doi:10.1006/bijl.2000.0484 [Google Scholar]
- Kemp D.J, Macedonia J.M. Structural ultraviolet ornamentation in the butterfly Hypolimnas bolina L. (Nymphalidae): visual, morphological and ecological properties. Aust. J. Zool. 2006;54:235–244. doi:10.1071/ZO06005 [Google Scholar]
- Kemp D.J, Rutowski R.L. Condition dependence, quantitative genetics, and the potential signal content of iridescent ultraviolet butterfly coloration. Evolution. 2007;61:168–183. doi: 10.1111/j.1558-5646.2007.00014.x. doi:10.1111/j.1742-4658.2007.00014.x [DOI] [PubMed] [Google Scholar]
- Kemp D.J, Vukusic P, Rutowski R.L. Stress-mediated covariance between nano-structural architecture and ultraviolet butterfly coloration. Funct. Ecol. 2006;20:282–289. doi:10.1111/j.1365-2435.2006.01100.x [Google Scholar]
- Knüttel H, Fiedler K. Host-plant-derived variation in ultraviolet wing patterns influences mate choice by male butterflies. J. Exp. Biol. 2001;204:2447–2459. doi: 10.1242/jeb.204.14.2447. [DOI] [PubMed] [Google Scholar]
- Kodric Brown A, Nicoletto P.F. Consensus among females in their choice of males in the guppy Poecilia reticulata. Behav. Ecol. Sociobiol. 1996;39:395–400. doi:10.1007/s002650050306 [Google Scholar]
- Lyytinen A, Lindstrom L, Mappes J. Ultraviolet reflection and predation risk in diurnal and nocturnal Lepidoptera. Behav. Ecol. 2004;15:982–987. doi:10.1093/beheco/arh102 [Google Scholar]
- Prum R.O, Quinn T, Torres R.H. Anatomically diverse butterfly scales all produce structural colours by coherent scattering. J. Exp. Biol. 2006;209:748–765. doi: 10.1242/jeb.02051. doi:10.1242/jeb.02051 [DOI] [PubMed] [Google Scholar]
- Robertson K.A, Monteiro A. Female Bicyclus anynana butterflies choose males on the basis of their dorsal UV-reflective eyespot pupils. Proc. R. Soc. B. 2005;272:1541–1546. doi: 10.1098/rspb.2005.3142. doi:10.1098/rspb.2005.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutowski R.L. Evidence for mate choice in a sulphur butterfly (Colias eurytheme) Zeit. Tierpsychol. 1985;70:103–114. [Google Scholar]
- Rutowski R.L. Male mate-locating behavior in the common eggfly, Hypolimnas bolina (Nymphalidae) J. Lepid. Soc. 1992;46:24–38. [Google Scholar]
- Shawkey M.D, Estes A.M, Siefferman L.M, Hill G.E. Nanostructure predicts intraspecific variation in ultraviolet-blue plumage colour. Proc. R. Soc. B. 2003;270:1455–1460. doi: 10.1098/rspb.2003.2390. doi:10.1098/rspb.2003.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberglied R.E. Visual communication and sexual selection among butterflies. In: Vane-Wright R.I, Ackery P.R, editors. The biology of butterflies. Academic Press; London, UK: 1984. pp. 207–223. [Google Scholar]
- Silberglied R.E, Taylor O.R. Ultraviolet reflection and its behavioral role in the courtship of the sulphur butterflies Colias eurytheme and C. philodice (Lepidoptera, Pieridae) Behav. Ecol. Sociobiol. 1978;3:203–243. doi:10.1007/BF00296311 [Google Scholar]
- Stride G.O. Further studies on the courtship behaviour of African mimetic butterflies. Anim. Behav. 1958;6:224–230. doi:10.1016/0003-3472(58)90055-1 [Google Scholar]
- Sweeney A, Jiggins C, Johnsen S. Polarized light as a butterfly mating signal. Nature. 2003;423:31–32. doi: 10.1038/423031a. doi:10.1038/423031a [DOI] [PubMed] [Google Scholar]
- Turner J.R.G. Why male butterflies are non-mimetic—natural-selection, sexual selection, group selection, modification and sieving. Biol. J. Linn. Soc. 1978;10:385–432. [Google Scholar]
- Vukusic P, Hooper I. Directionally controlled fluorescence emission in butterflies. Science. 2005;310:1151. doi: 10.1126/science.1116612. doi:10.1126/science.1116612 [DOI] [PubMed] [Google Scholar]
- Vukusic P, Sambles J.R. Photonic structures in biology. Nature. 2003;424:852–855. doi: 10.1038/nature01941. doi:10.1038/nature01941 [DOI] [PubMed] [Google Scholar]
- Wallace A.R. Macmillan & Co; London, UK: 1889. Darwinism: an exposition of the theory of natural selection, with some of its applications. [Google Scholar]
- Wiernasz D.C. Female choice and sexual selection of male wing melanin pattern in Pieris occidentalis (Lepidoptera) Evolution. 1989;43:1672–1682. doi: 10.1111/j.1558-5646.1989.tb02617.x. doi:10.2307/2409383 [DOI] [PubMed] [Google Scholar]