Abstract
It has been hypothesized that group-living mammals engage in reconciliation (post-conflict affiliation between former opponents) to reduce the disruptive costs of aggression and restore opponents' tolerance to baseline levels. Recipients of aggression are sometimes reluctant to tolerate the proximity of a recent opponent, however, in apparent fear that aggression will be renewed. In such cases, reconciliatory behaviour by the aggressor's close kin may substitute for direct reconciliation. We describe a playback experiment with free-ranging baboons (Papio hamadryas ursinus) that examines whether friendly behaviour by the aggressor's kin can substitute for direct reconciliation by the aggressor herself. In the test condition, female subjects who had recently been threatened heard the friendly grunt of one of their aggressor's relatives, mimicking kin-mediated vocal reconciliation. In the control condition, subjects heard the grunt of a dominant female from a different matriline. Subjects responded significantly more strongly in test than in control trials. Moreover, in the next hour they were significantly more likely to tolerate the proximity of both their aggressor and the relative whose grunt they had heard. In contrast, subjects' behaviour towards both control females and other members of their aggressor's matriline was unaffected. We conclude that kin-mediated vocal reconciliation can substitute for direct reconciliation in baboons.
Keywords: conflict management, direct reconciliation, kin-mediated reconciliation, baboon, recognition of others' kin
1. Introduction
Many group-living animals minimize the disruptive effects of aggression by reconciling after fights (see de Waal 1996; Wittig & Boesch 2003a). Post-conflict affiliation functions to restore recent opponents to baseline tolerance levels and may help to maintain group cohesion (Cords 1992; Cheney & Seyfarth 1997; Wittig & Boesch 2005). Reconciliation is thus potentially beneficial to both aggressors and their victims.
In order to engage in friendly reconciliatory behaviour, former opponents must come into close proximity soon after the aggressive interaction (see appendix in Wittig & Boesch 2005). However, subordinate victims may not always tolerate the close proximity of a former aggressor because approaches can result in renewed aggression (Aureli & van Schaik 1991; Wittig & Boesch 2003b). Thus, although reconciliation is potentially beneficial to both parties, dominant aggressors may not always be able to approach their victims to reconcile with them. This problem may be especially acute in ‘despotic’ monkey species characterized by strong matrilineal dominance hierarchies and unidirectional aggression (van Schaik 1983; Sterck et al. 1997). Female baboons (Papio hamadryas spp.) and rhesus macaques (Macaca mulatta), for example, reconcile at lower rates than more tolerant female lion-tailed macaques (Macaca silenus) or Tonkean macaques (Macaca tonkeana). Their dominance hierarchies are also comparatively more rigid (Thierry 1985, 2000; Silk et al. 1996).
There are at least two possible mechanisms that may facilitate reconciliation without requiring aggressors to come into close proximity with their victim. First, the aggressor can reconcile vocally. Female chacma baboons (Papio hamadryas ursinus) often grunt to their former victims soon after an aggressive interaction. Grunts seem to function as signals of benign intent, indicating that aggression is unlikely to be renewed (Cheney et al. 1995; Silk et al. 1996). In a playback experiment, Cheney & Seyfarth (1997) demonstrated that subordinate female baboons were more likely to tolerate the approach of their recent aggressor after hearing her grunt than after hearing no grunt or the grunt of another dominant female unrelated to the aggressor.
Second, kin may function as mediators, reconciling with the victim on behalf of their relative. Kin-mediated reconciliation might substitute for direct reconciliation when aggressors are not motivated to initiate friendly contact or when victims avoid their aggressor's approaches. Although apparent kin-mediated reconciliation occurs in a variety of monkey species (e.g. Cheney & Seyfarth 1989; Judge & Mullen 2005; see Das 2000 for review), it remains unclear whether it helps to restore the relationship between the opponents.
As in other non-human primate species, social interactions among chacma baboons (P. h. ursinus) are mediated by facial expressions, postures, gestures and a variety of different vocalizations (Cheney et al. 1995; Silk et al. 1996). When vegetation prohibits the use of gaze direction as a cue for inferring the intended target of a vocalization, baboons use a variety of other social cues when deciding whether or not to respond, including the signaller's identity, the call type and the nature of recent interactions. Playback experiments have demonstrated, for example, that if a baboon hears a recent opponent's threat-grunt she will often leave the immediate area (Engh et al. 2006b). If, however, she hears her opponent's ‘reconciliatory’ grunt, she is less likely to move away and often even approaches that female (Cheney & Seyfarth 1997). In both cases, the baboon acts as if she regards the call as directed at herself as a direct consequence of the recent interaction. In contrast, playback of an uninvolved female's call has little effect on subjects' behaviour. In this latter case, baboons behave as if they regard the call as directed towards someone else.
In this paper, we investigate the occurrence and function of kin-mediated vocal reconciliation among female chacma baboons. Using a playback experiment, subjects who had recently been threatened heard the grunt of one of their aggressor's close relatives (the ‘reconciling relative’), to mimic kin-mediated reconciliation. In the control condition, subjects heard the grunt of a dominant female from another matriline. On the assumption that subjects would treat the reconciling relative's grunt as a proxy for reconciliation with their aggressor, we predicted that subjects would be more likely to approach their opponent and tolerate her approaches after hearing the reconciling relative's grunt than after hearing the grunt of a female unrelated to their opponent.
2. Material and methods
(a) Study area and subjects
The study was conducted in the Moremi Game Reserve, in the Okavango Delta of Botswana, on a group of free-ranging chacma baboons. The habitat consists of seasonal flood plain interspersed with small ‘islands’ (Bulger & Hamilton 1987; Cheney et al. 2004). The group has been observed since 1978 and all animals are fully habituated to human observers on foot. At the time of these experiments (January–December 2005), the group contained approximately 70 individuals, including between 21–26 adult females and 5–10 adult males, 4–5 adolescent males and 2–9 adolescent females and 21–40 juveniles and infants. Experimental subjects were 13 adult females (less than 7 years). We conducted 16 matched-pair playback experiments including 13 different subjects.
As in other species of Old World monkey, female baboons form stable, linear dominance hierarchies based on the direction of approach–retreat interactions and aggression. Females assume ranks similar to their mothers', so that matrilineal relatives typically occupy adjacent ranks (Silk et al. 1999).
(b) Playback stimuli
Calls used as playback stimuli were recorded opportunistically from known individuals using Sennheisser ME88 microphones and NOMAD digital recorders. Sound files were saved in wav format and used within nine months from the time of the recording. After transferring the calls from the NOMAD to a laptop, we used CoolEdit software (Syntrillium, Phoenix, AZ) to ensure that the natural call sequences used as playback stimuli were high quality, without vocalizations from other baboons or masking background noise. All sequences were selected to be similar in call and bout length, rate and amplitude and to match the amplitude of naturally occurring calls. Calls were broadcast from a Bose Roommate II loudspeaker.
Female grunts are low amplitude, tonal vocalizations with a rich formant structure (Owren et al. 1997) that are given during friendly interactions. Females often grunt to each other during approaches or while handling infants. Acoustic analysis (Owren et al. 1997) and playback experiments (Cheney & Seyfarth 1997; Rendall et al. 1999) have shown that baboon grunts are individually distinctive to listeners.
All playback sequences consisted of three grunts and were similar in duration and amplitude. Different subjects usually heard different grunts (only one series of kin grunts and two series of non-kin grunts were broadcast twice each, but to different subjects). The mean duration of the reconciling relative's grunt sequence was 2.03±0.21 s; the mean duration of the unrelated female's sequence was 2.06±0.23 s. We used the grunts of nine females in test trials and seven females in control trials.
(c) Experimental protocol
The experiment followed a matched-pair design, with each subject appearing in two separate trials after being threatened (lunged at or chased) by the same dominant female. Playback experiments were conducted within 5 min of the original fight, as soon as the subject and her aggressor had separated without interacting or vocalizing and the subject was out of sight of the aggressor and all members of her matriline. The loudspeaker was hidden in vegetation at a distance of 5–8 m and at roughly 90° orientation from the subject, in the same direction from which the aggressor was last seen. All trials were conducted when the subject was either sitting or standing and not interacting with any other animal.
In the test trial, the subject heard the grunt of her aggressor's close relative (the reconciling relative). Close kin were defined as mothers, daughters or maternal sisters (r≥0.25), and matrilines as clusters of closely related females. In the control trial, the subject heard the grunt of another higher-ranking female belonging to a different matriline from the aggressor's. The order of presentation of test and control trials was alternated.
We used a SONY DCR-TRV25 digital video camera to record any changes in head position relative to the speaker in the 10 s before and 1 min after playback. We then followed the subject for 60 min to determine whether she came within the proximity (within 2 m) of her aggressor or any of her aggressor's matrilineal relatives. Whenever she did, we noted both the time and the nature of her encounter. The subject was defined as ‘tolerating’ the proximity of her aggressor if she either approached her aggressor or her aggressor's matrilineal relatives to within 2 m or did not move away when they approached to within 2 m. Such close proximity often leads to friendly interactions such as grooming and is only maintained when the subordinate is not avoiding the more dominant individual.
In addition to the playback follows, we conducted regular focal animal sampling (Altmann 1974) on each adult female. Focal animal observations lasted 10 min, and each female was sampled on average once a week for a period of 15 months (range 520–670 min). We used focal data to calculate natural frequencies of possible instances of vocal reconciliation by aggressors and aggressors' kin.
On the assumption that subjects would treat a grunt from the reconciling relative as a proxy for reconciliation with the aggressor herself, we predicted that subjects would look longer and more often towards the speaker, and perhaps even approach the speaker, in test than in control trials. We also predicted that, in the next hour, subjects would be more likely to tolerate the proximity of their aggressor and their aggressor's close relatives. Testing the last prediction, we compared latencies to tolerate the proximity. The latency was measured as the time between the playback stimuli and the first situation in which subjects tolerated the proximity of their aggressors or their aggressors' close relatives.
To minimize the possibility that the baboons would habituate to the playback stimuli, a maximum of two playback experiments was conducted daily. Playback experiments were never conducted within 2 h of each other, and the same subject never appeared in more than one playback experiment on the same day. Female baboons grunt to each other at very high rates (on average, once per 3 min; Cheney et al. 1995), so females heard playbacks of grunts at far lower rates than they heard naturally occurring grunts.
(d) Data analysis
Video films were analysed using Adobe Premier software. In coding experiments using a frame-by-frame method (15 frames per second), we measured three different responses: the duration of looking, number of looks towards the speaker in the first minute after the playback and whether or not the subject approached the speaker. A ‘look’ was defined as a head orientation directly towards the speaker. Movement was only recorded as an approach when it was directly towards the speaker and when it was the first move in any direction after the playback.
To analyse continuous measures of behavioural responses, we calculated average values for each of the subjects that were tested more than once (N=13). However, categorical bivariate measures had to be analysed per dyad (N=16).
To determine the legitimacy of testing three measures of behavioural response to the speaker after playback, we conducted a bivariate correlation test between the three dependent variables. All correlation factors were r<0.7, indicating minimal likelihood of multi-collinearity, and therefore no need to exclude any variables (Tabachnick & Fidell 2001). To determine the legitimacy of using parametric statistics, we conducted Kolmogorov–Smirnov one sample tests (Siegel & Castellan 1988) to test for normal distribution of the behavioural measures. Since none of the behavioural measures showed a normal distribution, we used non-parametric statistics.
Our post-trial follows yielded many tied results for two reasons. First, a tied result occurred by default whenever a subject failed to come into proximity of any of the relevant individuals in the ensuing hour in both conditions. These ‘default ties’ are a common occurrence in free-ranging animals, where interactions cannot be coerced. Second, ‘behavioural ties’ occurred when a subject's interactions with her aggressor, the reconciling relative or other members of the aggressor's matriline in the hour following playback were the same in both test and control conditions.
Owing to the small sample size, we conducted exact Wilcoxon matched-pairs signed-ranks and exact Sign test for categorical data (Mundry & Fischer 1998), to compare subjects' behaviour between trials (Siegel & Castellan 1988). Default ties were not considered in either test, which reduced the sample size accordingly. Since our hypotheses generated clear directional predictions, all tests were one-tailed (α=0.05). Bonferroni correction (indicated by ‘*’) was applied when variables were tested twice (α*=0.025).
Since ties jeopardize the power of classical non-parametric statistics, we also calculated the bias-corrected and accelerated (bca) confidence intervals of the differences between test and control conditions using bootstrap methods (DiCiccio & Efron 1996). The bca bootstrap confidence interval is recommended for general use, especially in cases when the assumption of normality is violated (Efron 1987). We calculated the difference in response between test and control conditions for each pair. We then generated the 90% CIs corresponding to a one-tailed test and the 95% CIs corresponding to a one-tailed test with Bonferroni correction for double testing, using bootstrap methods with 1000 replications. This method is equivalent to calculating a standard deviation for normally distributed, parametric data and takes ties into account. In tests where the bca confidence interval enclosed 0, we accepted the null hypothesis that there was no difference between test and control conditions. If it did not, we rejected the null hypothesis with α of 0.05.
In cases with few ties (less than 10%), our conclusions are drawn from the Wilcoxon or Sign test. Otherwise, we follow the results indicated by the bca confidence interval. In either case, however, we present both statistical results.
3. Results
(a) Natural frequency of vocal kin-mediated reconciliation
To determine the natural rate at which close kin grunted to the victims of their relative's aggression, we analysed the 106 aggressive interactions that involved aggressors with at least one close female relative and that occurred at least 5 min before a focal sample was terminated. In 20% of cases (N=21), a close relative of the aggressor grunted to the victim within 5 min after the dispute, with or without subsequent affiliative behaviour such as an embrace or grooming interaction. In contrast, the aggressor herself grunted to her victim following only 9% (N=10) of disputes.
(b) Responses to playback
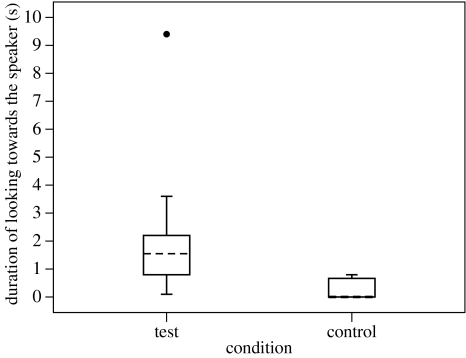
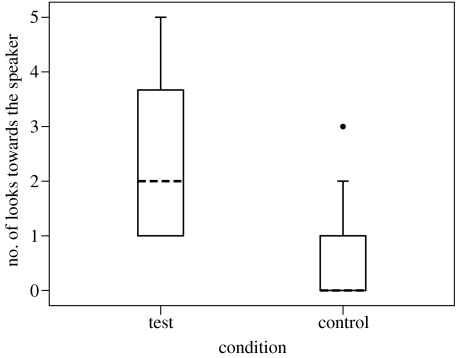
In playback trials, subjects' responses differed significantly between the two conditions. Specifically, subjects looked for longer (figure 1) and more times (figure 2) towards the speaker in the test than in the control condition (Duration of looking Wilcoxon test: N=13, T=5, p=0.0012; Bootstrap: p<0.05, no. 1 in table 1. Number of looks Wilcoxon test: N=13, T+=63, 2 ties, p=0.0024; Bootstrap: p<0.05, no. 2 in table 1). However, there was no difference between the two conditions in the likelihood that subjects would approach the speaker in the minute following playback (Sign test: N=16, k=1, 10 ties, p=0.109; Bootstrap: n.s., no. 3 in table 1).
Figure 1.
The duration that subjects looked towards the speaker in the first minute after playback in test and control trials (N=13). Median value is shown as a dashed line. Box-plots represent second and third quartile and error-bars represent the 95% confidence interval. Dots above or below the box-plots represent outliers.
Figure 2.
The number of times that subjects looked towards the speaker in the first minute after playback in test and control trials (N=13). Symbols are the same as given in figure legend 1.
Table 1.
Mean difference and bca confidence intervals of the differences between test and control conditions for each variable using bootstrap methods with 1000 replications. (MD, mean difference between test and control condition; CI, confidence interval.)
| no. | variable (unit) | N | MD | CI | |||
|---|---|---|---|---|---|---|---|
| % | min | max | p | ||||
| 1 | duration of looking towards the speaker (s) | 13 | 1.75 | 90 | 1.03 | 3.27 | <0.05 |
| 2 | number of looks towards the speaker (no.) | 13 | 1.78 | 90 | 1.06 | 2.58 | <0.05 |
| 3 | approaching the speaker (% yes versus no) | 16 | 25 | 90 | 0 | 50 | n.s. |
| 4 | latency to tolerate proximity (min) | ||||||
| a | of any member of the aggressor's matriline | 13 | −25.14 | 90 | −36.21 | −10.65 | <0.05 |
| b | of the aggressor | 13 | −17.91 | 90 | −28.38 | −7.46 | <0.05 |
| c | of the reconciling relative | 13 | −10.58 | 95 | −23.84 | −1.92 | <0.05 |
| d | of other kin of the aggressor | 13 | −5.47 | 90 | −19.38 | 5.72 | n.s. |
| e | of control female | 13 | −4 | 95 | −16.85 | 5.77 | n.s. |
| f | of reconciling relative versus control female | 13 | −9.6 | 95 | −19.08 | −0.05 | <0.05 |
(c) Interactions with the aggressor and her matriline
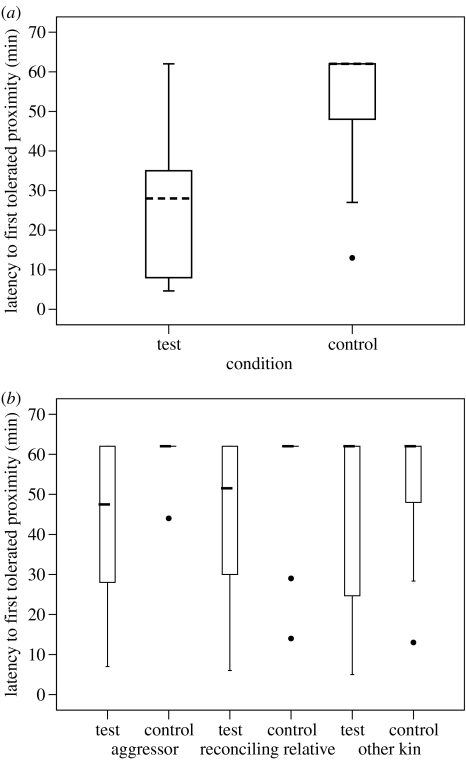
During the hour following playback, the subject and members of her aggressor's matriline (aggressor, reconciling relative and other kin) might or might not come into close proximity (less than 2 m). When they did, subjects showed a shorter latency to tolerate the proximity of these individuals after test trials than after control trials (figure 3a; Wilcoxon test: N=12, T=4, p=0.0017; Bootstrap: p<0.05, no. 4a in table 1). When the different members of the aggressor's matriline were considered separately, subjects showed a shorter latency to tolerate their aggressor's proximity after test trials than after control trials (figure 3b; Wilcoxon test: N=8, T=2, p=0.0117; Bootstrap: p<0.05, no. 4b in table 1). The bootstrap analysis also indicated that subjects showed a shorter latency to tolerate the reconciling relative's proximity (Bootstrap: p<0.05, no. 4c in table 1). This difference did not reach significance, however, in a Wilcoxon test (N=8, T=7, p*=0.0742). There was no difference between trial types in subjects' latency to tolerate proximity of other relatives of the aggressor's matriline (Wilcoxon test: N=8, T=10, p=0.1563; Bootstrap: n.s., no. 4d in table 1).
Figure 3.
The subjects' latency to first tolerate again proximity (a) of any member of their aggressor's matriline and (b) of a specific member of their aggressor's matriline in test and control trials (N=13). Symbols are the same as given in figure legend 1.
In total, subjects tolerated the proximity of either their aggressor (N=7), the reconciling relative (N=4) or other relatives (N=1) in 12 (75%) test trials, compared with only one (6%) control trial. In four of these 12 test trials, subjects also engaged in friendly behaviour, such as embracing or grooming, with their aggressor. One subject engaged in friendly behaviour with the reconciling relative. Subjects did not engage in friendly behaviour with any other members of the aggressor's matriline. Similarly, subjects never engaged in friendly behaviour with the aggressor or the reconciling relative in control trials.
Although hearing the grunt of a reconciling relative was correlated with changes in subjects' behaviour towards both the aggressor and the reconciling relative, hearing the grunt of an unrelated female did not alter subjects' behaviour towards that female. After hearing the control female's grunt, subjects did not show a shorter latency to tolerate that female's proximity (Wilcoxon test: N=5, T=5, p*=0.3125; Bootstrap: n.s., no. 4e in table 1). Indeed, subjects showed a shorter latency to tolerate the proximity of the reconciling relative after hearing her grunt than to tolerate the proximity of the unrelated female after hearing hers (Wilcoxon test: N=9, T=5, p*=0.0195; Bootstrap: p<0.05, no. 4f in table 1).
In sum, the reconciliatory grunt of the aggressor's close kin had the strongest effect on the subject's behaviour towards the aggressor, the second strongest effect on the subject's behaviour towards the reconciling relative and the weakest effect on the other members of the aggressor's matriline. Finally, hearing the grunt of a control female had no effect on subjects' behaviour towards any female.
4. Discussion
Upon hearing the grunt of their aggressor's close relative, female baboons looked towards the speaker more often and for a longer duration than upon hearing the grunt of a female unrelated to their aggressor. Moreover, in the hour following playback of the reconciling relative's grunt, subjects' latency to tolerate their aggressor's proximity was significantly shorter. Subjects responded as if they assumed that the reconciling relative's vocalization was directed at them and was causally related to the recent fight. They appeared to regard the relative's grunt as a proxy for reconciliation with the aggressor herself. Subjects' disposition towards the reconciling relative was also affected; their latency to tolerate the reconciling relative's proximity was significantly shorter than their latency to tolerate the proximity of the control female.
Although the grunt of the reconciling relative functioned to reconcile subjects with their aggressor, subjects did not generalize their response towards all members of the matriline. Their behaviour towards other members of the aggressor's matriline was relatively unaffected. These results provide some evidence that, while baboons recognize other individuals' membership in particular matrilines (Cheney & Seyfarth 1999; Bergman et al. 2003), they nonetheless discriminate among the different members that comprise them. They react as if they do not treat all the members of a matriline as equivalent or ‘mutually substitutable’ (cf. Schusterman & Kastak 1998; Schusterman et al. 2003).
When assessing the intended recipient of another individual's vocalization, female baboons appear to take into account the signaller's identity, the type of vocalization given, the nature of recent interactions and the signaller's relationship with her recent behavioural partners. Vocalization type is crucial in guiding females' responses towards former opponents. While grunts function to reconcile, threat-grunts are perceived as a renewal of aggression (Engh et al. 2006b; Wittig et al. 2007). Furthermore, females do not simply alter their disposition towards any individual whose call they hear, because in these experiments subjects' behaviour towards control females was unaffected by these females' friendly grunts. Perhaps because they had not recently interacted with the control female, subjects seemed to interpret that female's grunt as irrelevant to the recent fight and directed at someone else (see also Engh et al. 2006b). In contrast, even though they had also not recently interacted with the reconciling relative, they treated this female's grunt as relevant to the dispute. Apparently, the relative's close bond with the aggressor was sufficient to cause subjects to infer that the grunt must be directed at them.
The proximate mechanisms that motivate a female baboon to reconcile with her relative's victim are not immediately obvious. It is unlikely that baboons empathize in the sense of projecting their own mental states onto others, because baboons and other monkeys appear unable to attribute mental states different from their own to others (reviewed by: Cheney & Seyfarth 1990; Tomasello & Call 1997; Silk in press). Similarly, it seems unlikely that baboons reconcile with other females' opponents because friendly contact alleviates the general anxiety that arises as a consequence of witnessing aggression, because not all bystanders are equally likely to be friendly. Moreover, the victims of disputes do not accept friendly grunts from females unrelated to their opponent as an indication of reconciliation. Only the relative's grunt is relevant.
Kin-mediated reconciliation may ultimately be linked to the inclusive fitness of the reconciling relative. If disputes remain unreconciled, tolerance may remain disrupted between both opponents and their matrilines, and group cohesion may be weakened. Such loss of cohesion may prove costly in areas of high predation (Janson 1992) or during inter-group competition for resources (Wrangham 1980; Cheney 1992). Regardless of their dominance rank, group life is essential for all female baboons, and more socially integrated females experience higher infant survival (Silk et al. 2003) and reduced stress (Beehner et al. 2005; Engh et al. 2006a). In the Okavango Delta, females' reproductive success is determined primarily by predation and infanticide (Cheney et al. 2004), and females can diminish the deleterious effects of these two selective pressures by establishing and maintaining close bonds with kin, adult males and other adult females. Reconciliation serves the important function of minimizing and ameliorating the disruptive effects of aggression and restoring tolerance among females.
In baboons, however, direct reconciliation between aggressors and their victims is relatively rare, occurring after only 9–13% of conflicts (Silk et al. 1996). This low frequency may arise in part because subordinate victims avoid their opponents after a dispute in apparent fear that aggression will be renewed. Results from these playback experiments suggest that aggressors' kin can ameliorate the potentially disruptive effects of aggression by reconciling on their relatives' behalf. Our observations indicate that females grunt to the victims of their relatives' aggression following 20% of disputes, a rate double that of direct reconciliation. Taken together, this triples the frequency of reconciliation in baboons to 30–40% of all conflicts, a level similar to that found in ‘egalitarian’ species, such as the moor macaques (Macaca maurus; 42%; Matsumura 1996) and chimpanzees (Pan troglodytes; range: 19.2–32.1%; Arnold & Whiten 2001; Kutsukake & Castles 2004; Wittig & Boesch 2005). If similar kin-mediated reconciliation occurs at equal frequencies in other despotic monkey species, the apparent variation in reconciliatory frequencies between despotic and more tolerant species may disappear (Thierry 2000; see Appendix A of Aureli & de Waal 2000).
In chimpanzees, third-party post-conflict affiliation by an uninvolved bystander towards the victim of aggression has operationally been termed ‘consolation’ (de Waal & van Roosmalen 1979). Although consolation has previously been thought to be unique to apes for both cognitive and social reasons (de Waal & Aureli 1996; Watts et al. 2000; Preston & de Waal 2002), there appear to be many behavioural parallels between consolation in chimpanzees and kin-mediated reconciliation in monkeys (Cheney & Seyfarth 1989; Call et al. 2002). Indeed, just as consolation is hypothesized to substitute for reconciliation in apes (Wittig & Boesch 2003b; Palagi et al. 2006), so does kin-mediated reconciliation function as a substitute for direct reconciliation in baboons.
In conclusion, female baboons who are the victims of aggression appear to accept a reconciliatory grunt by a relative of their aggressor as a proxy for reconciliation with the aggressor herself. A friendly signal from a non-relative does not serve this function, and reconciliation is extended to the opponent and the stand-in relative, but probably not to other members of the opponent's matriline. This hypothesis of kin-mediated vocal reconciliation assumes that victims can recognize other females' kin (or close relations) and make inferences about the intended target of a vocalization. Both of these assumptions are supported by field experiments on baboons (e.g. Cheney & Seyfarth 1999; Engh et al. 2006b). Kin-mediated reconciliation, however, does not require an ability to attribute mental states to others.
Acknowledgments
We thank the Office of the President and the Department of Wildlife and National Parks of the Republic of Botswana for permission to conduct research in the Moremi Reserve. Alec Mokopi and Mokopi Mokopi provided invaluable assistance in the field with data collection and with conducting the experiments. We are also very grateful to Julia Fischer, Julia Lehmann and Simone Pika, and to Daniel Stahl for statistical advice. Research was supported by the National Institutes of Health grant no. MH62249, by the Deutsche Forschungsgemeinschaft (DFG) research fellowship WI 2637/2-1 and by the Department of Linguistics of the MPI EVA, Leipzig. This research was reviewed and approved by the Animal Care and Use Committee at the University of Pennsylvania. We would like to dedicate this research to M. Mokopi, who died recently.
References
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Arnold K, Whiten A. Post-conflict behaviour of wild chimpanzees (Pan troglodytes schweinfurthii) in the Budongo Forest, Uganda. Behaviour. 2001;138:649–690. doi:10.1163/156853901316924520 [Google Scholar]
- Aureli F, de Waal F.B.M. University of California Press; Berkley, CA: 2000. Natural conflict resolution. [Google Scholar]
- Aureli F, van Schaik C.P. Post-conflict behaviour in long-tailed macaques (Macaca fascicularis) Ethology. 1991;89:101–114. [Google Scholar]
- Beehner J.C, Bergman T.J, Cheney D.L, Seyfarth R.M, Whitten P.L. The effect of new alpha males on female stress in free-ranging baboons. Anim. Behav. 2005;69:1211–1221. doi:10.1016/j.anbehav.2004.08.014 [Google Scholar]
- Bergman T.J, Beehner J.C, Cheney D.L, Seyfarth R.M. Hierarchical classification by rank and kinship in baboons. Science. 2003;302:1234–1236. doi: 10.1126/science.1087513. 10.1126/science.1087513 [DOI] [PubMed] [Google Scholar]
- Bulger J, Hamilton W.J. Rank and density correlates of inclusive fitness measures in a natural chacma baboon (Papio ursinus) troop. Int. J. Primatol. 1987;8:635–650. [Google Scholar]
- Call J, Aureli F, de Waal F.B.M. Postconflict third-party affiliation in stumptailed macaques. Anim. Behav. 2002;63:209–216. doi:10.1006/anbe.2001.1908 [Google Scholar]
- Cheney D.L. Intragroup cohesion and intergroup hostility—the relation between grooming distributions and intergroup competition among female primates. Behav. Ecol. 1992;3:334–345. doi:10.1093/beheco/3.4.334 [Google Scholar]
- Cheney D.L, Seyfarth R.M. Reconciliation and redirected aggression in vervet monkeys Cercopithecus aethiops. Behaviour. 1989;110:258–275. [Google Scholar]
- Cheney D.L, Seyfarth R.M. University of Chicago Press; Chicago, IL: 1990. How monkeys see the world. [Google Scholar]
- Cheney D.L, Seyfarth R.M. Reconciliatory grunts by dominant female baboons influence victims' behaviour. Anim. Behav. 1997;54:409–418. doi: 10.1006/anbe.1996.0438. doi:10.1006/anbe.1996.0438 [DOI] [PubMed] [Google Scholar]
- Cheney D.L, Seyfarth R.M. Recognition of other individuals' social relationships by female baboons. Anim. Behav. 1999;58:67–75. doi: 10.1006/anbe.1999.1131. doi:10.1006/anbe.1999.1131 [DOI] [PubMed] [Google Scholar]
- Cheney D.L, Seyfarth R.M, Silk J.B. The role of grunts in reconciling opponents and facilitating interactions among adult female baboons. Anim. Behav. 1995;50:249–257. doi:10.1006/anbe.1995.0237 [Google Scholar]
- Cheney D.L, et al. Factors affecting reproduction and mortality among baboons in the Okavango Delta, Botswana. Int. J. Primatol. 2004;25:401–428. doi:10.1023/B:IJOP.0000019159.75573.13 [Google Scholar]
- Cords M. Post-conflict reunions and reconciliation in long-tailed macaques. Anim. Behav. 1992;44:57–61. doi:10.1016/S0003-3472(05)80754-7 [Google Scholar]
- Das M. Conflict management via third parties. In: Aureli F, de Waal F.B.M, editors. Natural conflict resolution. California University Press; Berkeley, CA: 2000. pp. 263–280. [Google Scholar]
- de Waal F.B.M. Conflict as negotiation. In: McGrew W.C, Marchant L.F, Nishida T, editors. Great ape societies. Cambridge University Press; Cambridge, UK: 1996. pp. 159–172. [Google Scholar]
- de Waal F.B.M, Aureli F. Consolation, reconciliation, and a possible cognitive difference between macaques and chimpanzees. In: Russon A.E, Bard K.A, Parker S.T, editors. Reaching into thought: the minds of the great apes. Cambridge University Press; Cambridge, UK: 1996. pp. 80–110. [Google Scholar]
- de Waal F.B.M, van Roosmalen A. Reconciliation and consolation among chimpanzees. Behav. Ecol. Sociobiol. 1979;5:55–66. doi:10.1007/BF00302695 [Google Scholar]
- DiCiccio T.J, Efron B. Bootstrap confidence intervals (with discussion) Stat. Sci. 1996;11:189–228. doi:10.1214/ss/1032280214 [Google Scholar]
- Efron B. Better bootstrap confidence intervals. J. Am. Stat. Assoc. 1987;82:171–200. doi:10.2307/2289144 [Google Scholar]
- Engh A.L, Beehner J.C, Bergman T.J, Whitten P.L, Hoffmeier R.R, Seyfarth R.M, Cheney D.L. Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus) Proc. R. Soc. B. 2006a;273:707–712. doi: 10.1098/rspb.2005.3378. doi:10.1098/rspb.2005.3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh A.L, Hoffmeier R.R, Cheney D.L, Seyfarth R.M. Who me? Can baboons infer the target of vocalizations? Anim. Behav. 2006b;71:381–387. doi:10.1016/j.anbehav.2005.05.009 [Google Scholar]
- Janson C.J. Evolutionary ecology of primate social structure. In: Smith E.A, Winterhalder B, editors. Evolutionary ecology and human behavior. Adline De Gruyter; New York, NY: 1992. pp. 95–130. [Google Scholar]
- Judge P.G, Mullen S.H. Quadratic postconflict affiliation among bystanders in a hamadryas baboon group. Anim. Behav. 2005;69:1345–1355. doi:10.1016/j.anbehav.2004.08.016 [Google Scholar]
- Kutsukake N, Castles D.L. Reconciliation and post-conflict third-party affiliation among wild chimpanzees in the Mahale Mountains, Tanzania. Primates. 2004;45:157–165. doi: 10.1007/s10329-004-0082-z. doi:10.1007/s10329-004-0082-z [DOI] [PubMed] [Google Scholar]
- Matsumura S. Postconflict affiliative contacts between former opponents among wild moor macaques (Macaca maurus) Am. J. Primatol. 1996;38:211–219. doi: 10.1002/(SICI)1098-2345(1996)38:3<211::AID-AJP2>3.0.CO;2-1. doi:10.1002/(SICI)1098-2345(1996)38:3<211::AID-AJP2>3.0.CO;2-1 [DOI] [PubMed] [Google Scholar]
- Mundry R, Fischer J. Use of statistical programs for nonparametric tests of small samples often leads to incorrect P values: examples from Animal Behaviour. Anim. Behav. 1998;56:256–259. doi: 10.1006/anbe.1998.0756. doi:10.1006/anbe.1998.0756 [DOI] [PubMed] [Google Scholar]
- Owren M.J, Seyfarth R.M, Cheney D.L. The acoustic features of vowel-like grunt calls in chacma baboons, Papio cynocephalus ursinus: implications for production processes and functions. J. Acoust. Soc. Am. 1997;101:2951–2963. doi: 10.1121/1.418523. doi:10.1121/1.418523 [DOI] [PubMed] [Google Scholar]
- Palagi E, Cordoni G, Borgognini Tarli S. Possible roles of consolation in captive chimpanzees (Pan troglodytes) Am. J. Phys. Anthropol. 2006;129:105–111. doi: 10.1002/ajpa.20242. doi:10.1002/ajpa.20242 [DOI] [PubMed] [Google Scholar]
- Preston S.D, de Waal F.B.M. Empathy: its ultimate and proximate bases. Behav. Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. doi:10.1017/S0140525X02000018 [DOI] [PubMed] [Google Scholar]
- Rendall D, Seyfarth R.M, Cheney D.L, Owren M.J. The meaning and function of grunt variants in baboons. Anim. Behav. 1999;57:583–592. doi: 10.1006/anbe.1998.1031. doi:10.1006/anbe.1998.1031 [DOI] [PubMed] [Google Scholar]
- Schusterman R.J, Kastak D. Functional equivalence in a California sea lion: relevance to social and communicative interactions. Anim. Behav. 1998;55:1087–1095. doi: 10.1006/anbe.1997.0654. doi:10.1006/anbe.1997.0654 [DOI] [PubMed] [Google Scholar]
- Schusterman R.J, Reichmuth Kastak C, Kastak D. Equivalence classification as an approach to social knowledge: from sea lions to simians. In: de Waal F.B.M, Tyack P.L, editors. Animal social complexity. Harvard University Press; Cambridge, MA: 2003. pp. 179–206. [Google Scholar]
- Siegel S, Castellan N.J.J. 2nd edn. McGraw-Hill, Inc; New York, NY: 1988. Nonparametric statistics for the behavioral sciences. [Google Scholar]
- Silk, J. B. In press. Empathy, sympathy, and prosocial preference in primates. In Oxford handbook of evolutionary psychology (eds R. I. M. Dunbar & L. Barrett).
- Silk J.B, Cheney D.L, Seyfarth R.M. The form and function of post-conflict interactions between female baboons. Anim. Behav. 1996;52:259–268. doi:10.1006/anbe.1996.0171 [Google Scholar]
- Silk J.B, Seyfarth R.M, Cheney D.L. The structure of social relationships among female savannah baboons in Moremi Reserve, Botswana. Behaviour. 1999;136:679–703. doi:10.1163/156853999501522 [Google Scholar]
- Silk J.B, Alberts S.C, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1231–1234. doi: 10.1126/science.1088580. doi:10.1126/science.1088580 [DOI] [PubMed] [Google Scholar]
- Sterck E.H.M, Watts D.P, van Schaik C.P. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 1997;41:291–309. doi:10.1007/s002650050390 [Google Scholar]
- Tabachnick B.G, Fidell L.S. 4th edn. Allyn & Bacon; London, UK: 2001. Using multivariate statistics. [Google Scholar]
- Thierry B. Patterns of agonistic interactions in three species of macaque (Macaca mulatta, M. fascicularis, M. tonkeana) Aggressive Behav. 1985;11:223–233. doi: 10.1016/0376-6357(85)90105-6. [DOI] [PubMed] [Google Scholar]
- Thierry B. Conflict management patterns across macaque species. In: Aureli F, de Waal F.B.M, editors. Natural conflict resolution. California University Press; Berkeley, CA: 2000. pp. 106–128. [Google Scholar]
- Tomasello M, Call J. Oxford University Press; New York, NY: 1997. Primate cognition. [Google Scholar]
- van Schaik C.P. Why are diurnal primates living in groups? Behaviour. 1983;87:120–144. [Google Scholar]
- Watts D.P, Colmenares F, Arnold K. Redirection, consolation, and male policing. In: Aureli F, de Waal F.B.M, editors. Natural conflict resolution. California University Press; Berkeley, CA: 2000. pp. 281–301. [Google Scholar]
- Wittig R.M, Boesch C. ‘Decision-making’ in conflicts of wild chimpanzees (Pan troglodytes): an extension of the relational model. Behav. Ecol. Sociobiol. 2003a;54:491–504. doi:10.1007/s00265-003-0654-8 doi:10.1007/s00265-003-0696-y. [Google Scholar]
- Wittig R.M, Boesch C. The choice of post-conflict interactions in wild chimpanzees (Pan troglodytes) Behaviour. 2003b;140:1527–1559. doi:10.1163/156853903771980701 [Google Scholar]
- Wittig R.M, Boesch C. How to repair relationships—reconciliation in wild chimpanzees (Pan troglodytes) Ethology. 2005;111:736–763. doi:10.1111/j.1439-0310.2005.01093.x [Google Scholar]
- Wittig, R. M., Crockford, C., Seyfarth, R. M. & Cheney, D. L. 2007 Vocal alliances in chacma baboons (Papio hamadryas ursiunus). Behav. Ecol. Sociobiol. (doi:10.1007/s00265-006-0319-5)
- Wrangham R.W. An ecological model of female-bonded primate groups. Behaviour. 1980;75:262–300. [Google Scholar]