Abstract
The ability of animals to gather information about their social and physical environment is essential for their ecological function. Odour cues are an important component of this information gathering across taxa. Recent laboratory studies have revealed the importance of flexible chemical cues in facilitating social recognition of fishes. These cues are known to be mediated by recent habitat experience and fishes are attracted to individuals that smell like themselves. However, to be relevant to wild populations, where animals may move and forage freely, these cues would have to be temporally flexible and allow spatial resolution. Here, we present data from a study of social recognition in wild populations of three-spined sticklebacks (Gasterosteus aculeatus). Focal fish preferentially associated with conspecifics from the same habitat as themselves. These preferences were changed and updated following translocation of the focal fish to a different site. Further investigation revealed that association preferences changed after 3 h of exposure to different habitat cues. In addition to temporal flexibility, the cues also allowed a high degree of spatial resolution: fish taken from sites 200 m apart produced cues that were sufficiently different to enable the focal fish to discriminate and associate with fish captured near their own home site. The adaptive benefits of this social recognition mechanism remain unclear, though they may allow fish to orient within their social environment and gain current local information.
Keywords: odour, olfactory, schooling, social organization
1. Introduction
Animals often organize themselves into social groups or into territorial assemblages; local organization also occurs within these structures, so a group of social animals is rarely just a random subset of the local population (see Krause & Ruxton 2002). This social organization is underpinned by recognition. Animals use a suite of different cues to obtain information about their environment and to recognize the individuals that they encounter (Liebert & Starks 2004; Mateo 2004). In taxa ranging from mammals to social insects, chemical cues are of enormous importance, allowing both general and specific individual recognition (Wyatt 2003). Further to this, studies have shown how habitat use and diet can directly affect chemical cues. For example, diet affects chemical communication between rodents (Brown et al. 1996; Schellink et al. 1997). Similarly, environmental and diet cues are important in nest mate recognition in social insects (Crosland 1989; Liang & Silverman 2000), even overriding genetic cues (Beye et al. 1997, 1998).
Chemical cues are of particular relevance to aquatic animals owing to the properties of water as a solvent and a medium to disperse such cues, and owing to limitations on vision at depth and in complex or turbid environments. In freshwater fishes, social recognition is achieved using a combination of visual and chemical cues (Brown & Smith 1994). Vision is of primary importance to fishes in short-range detection (Douglas & Hawryshyn 1990). However, over greater distances than a few body lengths, they may gather information using their olfactory and gustatory senses. Chemical cues are of great importance in social recognition, both for shoaling fishes (Ward et al. 2002, 2004) and in dominance hierarchies (Todd et al. 1967). Further to this, odour of an individual is strongly influenced by both recent habitat use and diet (Ward et al. 2004) and this mediates association preferences; fish are socially attracted to individuals that smell similar to themselves, suggesting chemical self-referencing (Mateo 2004; Ward et al. 2004, 2005).
Many social animals are choosy about group mates, often forming long-term associations with particular, unrelated individuals (Griffiths & Ward 2006). ‘Familiarity’, as this phenomenon is known, acts to enhance the benefits of grouping. In fishes, shoals composed of familiar individuals are more cohesive, potentially increasing the shoal's anti-predator function (Chivers et al. 1995). Shoals of familiars out-perform random shoals in foraging tasks (Ward & Hart 2005).
Familiarity is, by definition, reliant upon social recognition, which may be achieved by learning the individual identities of frequently encountered individuals over time (Griffiths & Magurran 1997a), or by a more general recognition of some characteristics common to members of the local environment, such as habitat-specific chemical cues (Ward et al. 2004). Sticklebacks discount social experience when choosing whom to associate with, instead they prefer to shoal with previously unencountered fish that smell the same as themselves (Ward et al. 2005), suggesting that, in the laboratory, familiarity in sticklebacks is mediated by general recognition.
A criticism of laboratory-based investigations into social recognition using chemical cues is that experimental fish are conditioned to particular water chemistry conditions and to a specific diet, thereby producing extreme differences in the cues that they ultimately express. This is in contrast to the natural environment, where fishes are theoretically free to move around between different microhabitats and feed widely. This could have the effect of minimizing any differences between the cues that individuals produce, yet if habitat- and diet-based cues are to be relevant to free-ranging fishes, they would have to be specific enough to allow spatial resolution, while being flexible enough to respond to changes in recent habitat experience of a fish. We tested the ability of free-ranging fish to discriminate between individuals on the basis of cues resulting from recent habitat experience. We tested the ability of choosing fish to distinguish between sample groups of conspecifics taken from different parts of the same drainage system. Finally, we investigated the temporal flexibility of the cues. We predicted that fish would discriminate on the basis of the cues, preferring to associate with individuals taken from the same local environment.
2. Material and methods
(a) Experimental fish
All fish used were obtained from the estuary, or water courses connected to the estuary, of the Great Eau at Saltfleet, Lincolnshire, UK (Grid reference TF 464 935 GB) during September and October 2005. We collected fish from three different sites: site 1 is in the estuary 100 m downstream from where the drainage channel enters the estuary; site 2 is in the drainage channel 100 m upstream of point where it meets the estuary; and site 3 is also in the drainage ditch 200 m upstream of site 2. The three sites are hence set at 200 m intervals. The drainage channel communicates directly with the estuary through a sluice gate that opens at low tide. Both environments feature flowing water. We repeatedly measured physical and chemical characteristics at each site (table 1).
Table 1.
Measured environmental variables at each of the three sampling sites.
| channel depth (m) | channel width (m) | vegetation | flow rate (cm s−1) | salinity (specific gravity) | pH | |
|---|---|---|---|---|---|---|
| site 1 (estuary) | 2–4 | 15 | no aquatic macrophytes. Sparse overhanging vegetation | −8–10 | 1.001–1.024 | 7.2–7.5 |
| site 2 (ditch) | 0.6–0.8 | 2 | no aquatic macrophytes, 80–100% cover provided by floating algal mat. Cover provided by tall vegetation on steep banks | 1–10 | 1.006–1.015 | 7.5 |
| site 3 (ditch) | 0.3–0.5 | 1.5 | no aquatic macrophytes. No floating algal mat. Sparse overhanging vegetation | 1–10 | 1.005–1.013 | 7.3–7.5 |
We sorted fish by body length to avoid the confounding effects of size-assortative shoaling (Ward & Krause 2001), using juvenile fish of approximately 30 mm body length, measured from the tip of the snout to the end of the caudal peduncle. All fish were released at their site of capture following completion of the experiments.
(b) Experimental design
To test the association preference of sticklebacks, we carried out binary choice shoaling tests. We divided a tank measuring 45×25×25 cm (l×w×d) into three sections along its longest axis, such that there was a central section measuring 29×25×25 cm (l×w×d) flanked by two outer sections, each measuring 8×25×25 cm (l×w×d). We used transparent, perforated plastic dividers to allow visual and chemical communication between all sections. Water depth was 200 mm and we provided a gravel substrate. We drew a line outside the glass to divide the central compartment into two zones. The test tank was located in a shelter at the water's edge, allowing us to minimize the time between fish capture and experimentation. The test tank, with the exception of a central viewing panel, was covered with black plastic to minimize stress to the occupants.
At the start of each batch of trials, we added water to the test tank, half of which was taken from each of the sites to be investigated to allow an intermediate aquatic environment for the experiments. We added two shoals of 10 freshly caught and size-matched stimulus fish, one to each of the outer compartments. The stimulus fish were allowed to acclimatize for 30 min before we introduced a solitary focal fish, again matched in size to the stimulus shoals, to the centre of the main compartment. During this time, focal fish were caught and held in buckets containing water taken from the site where they were caught. Once the acclimatization period had elapsed, we added a focal fish to the centre of the test tank. The focal fish was allowed to acclimatize for 2 min after which we recorded the amount of time spent by the focal fish within each half of the central compartment as a measure of shoaling preference for a further 2 min. Following this, we removed the focal fish very carefully with a clean net and allowed 1 min to elapse before adding the next focal fish. In designing the experiments, we were mindful of the potential for the effect of exposure to the mix of water in the test tank to degrade the cues provided by the stimulus fish. Selecting a 2 min observation period in conjunction with the initial 2 min acclimatization period and the minute following removal of each focal fish enabled us to keep each replicate for approximately 5 min. To be sure that the shoaling preferences of focal sticklebacks measured over a 2 min experimental period would be representative of their shoaling preferences measured over a longer period of time, we ran control tests using two groups of 20 sticklebacks in the laboratory, using the same binary choice procedure detailed previously. One group was tested for 5 min and the other for 10 min. In each case, we recorded the preferences of the focal fish 2 min into the trial and again at the end of the trial, using this information, we were then able to measure the ability of the results after 2 min to predict the results at the end of the trial. A 2 min acclimatization period proved sufficient for almost all of the focal fish to be swimming normally, probably because fish were handled for very little time following capture and were subsequently maintained in their own water. On the three occasions that the focal fish exhibited stress, we discontinued the trial and changed the focal fish. Each focal fish was used only once. The fish in the stimulus shoals swam actively throughout; we detected no signs of stress, such as seeking refuge on the bottom or darting frantically around. The stimulus shoals and the water in each aquarium were replaced following the completion of eight trials. The continual use of stimulus fish for eight consecutive trials not only enabled us to restrict the number of experimental animals, but also meant that we replicated our stimulus. To avoid excessive bias, we conducted tests on more than one stimulus group within each treatment and analysed the preference of focal fish between stimulus groups. We allocated stimulus shoals at random to either side of the experimental aquarium. To minimize the possibility of experimental bias, the trials were conducted blind: the person observing the behaviour of the focal fish did not know the identity of the stimulus shoals and relayed the data to a second experimenter who recorded the information.
(c) Spatial resolution
(i) Coarse-scale habitat differentiation
In our first experiment, we took one batch of experimental fish from site 2 (in the drainage channel) and the second batch from site 1 (in the estuary) and measured the association preferences of focal fish taken from each of the two sites. We alternated the focal fish for each trial, using site 2 fish then site 1 fish, then site 2 again until we had completed a total of 24 replicates. In our second experiment, we caught a batch of experimental fish from site 2 and transplanted them into three separate holding nets measuring approximately 1.5 m in length and 0.5 m in diameter at site 1, where they were held for 14 h, during which time they were able to feed from the substrate through the nets. We then measured the preference of transplanted focal fish for stimulus shoals taken from sites 2 and 1, conducting a total of 16 replicates.
(ii) Fine-scale habitat differentiation
In our third experiment, we took one batch of experimental fish from site 2 and a further batch of experimental fish from site 3. We then measured the preference of focal fish taken from each site, again alternating the focal fish as described above until we had conducted a total of 24 replicates.
(d) Temporal flexibility
For our fourth and final experiment, we took one batch of experimental fish from site 1 and a further batch from site 2. To manipulate the habitat experience of focal fish, we held the focal fish from each site in separate containers filled with an equal mix of water from the two sites prior to experimentation for 1, 3–4 and 7–8 h. In each case, once this period had elapsed, we measured the preference of focal fish using stimulus fish taken freshly from each of the two environments. We conducted a total of 24 replicates for each of the three time delay treatments.
(e) Data analysis
We used non-parametric statistics to analyse the results of the shoaling preference tests throughout. We subtracted the time spent shoaling with stimulus shoal ‘b’ from the time spent shoaling with stimulus shoal ‘a’ and compared the resultant values with the null expectation of 0. Where we performed multiple tests within a treatment, we applied a sequential Bonferroni technique to recalculate significance levels (Holm 1979; Rice 1989). To test for bias in our datasets, we also compared the strength of the shoaling preferences of focal fish from different sites for their respective matching stimulus shoals, and the response of focal fish towards different stimulus shoals.
3. Results
(a) Control: the use of a 2 min test period
Two minute observations of the shoaling preferences of focal fish were able to predict their shoaling preferences at the end of a longer trial, both in the 5 min (linear regression: r2=0.26, n=20, p=0.013) and in the 10 min trial control groups (linear regression: r2=0.21, n=20, p=0.023).
(b) Spatial resolution
(i) Coarse-scale habitat differentiation
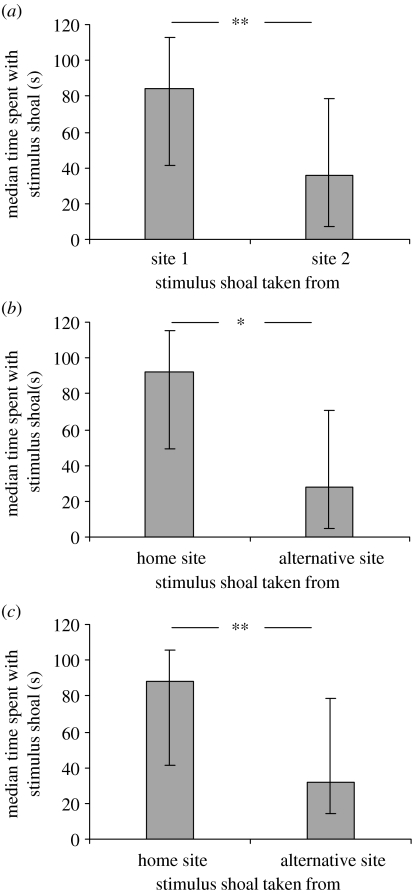
The focal fish preferentially associated with a stimulus shoal taken from their home environment over a stimulus shoal from the alternative (Wilcoxon test: Z=2.8, n=24, p=0.005; figure 1a). There was no difference in the strength of the shoaling preferences of focal fish from either habitat (Mann–Whitney U-test: U12,12=0.96, p=0.331) or in the response of focal fish to the three stimulus groups (Kruskal–Wallis ANOVA: χ22,21=1.1, p=0.564). Focal fish that were transplanted into the alternative environment preferentially associated with a stimulus shoal from their new environment over a stimulus shoal from their former environment (Wilcoxon test: Z=2.4, n=16, p=0.019; see figure 1b). There was no difference in the response of focal fish to the two stimulus groups (Kruskal–Wallis ANOVA: χ1,142=0.47, p=0.492).
Figure 1.
Median time±quartiles spent by focal fish with each of two stimulus shoals: (a) Focal fish taken from sites 1 and 2 were given a choice between stimulus fish from their home site and stimulus fish from the alternative site, n=24; (b) Focal fish taken from site 2 were transplanted to site 1 overnight before being given a choice between stimulus fish from their site 1 and stimulus fish from site 2, n=16 and (c) Focal fish taken from sites 2 and 3 were given a choice between stimulus fish from their home site and stimulus fish from the alternative site, n=24. Differences in time spent shoaling by focal fish with the two stimulus shoals in binary choice tests were derived by subtracting time spent with shoal ‘b’ from time spent with shoal ‘a’ and comparing the results against a null expectation of 0. Significant differences are denoted as follows: *p<0.05; **p<0.01.
(ii) Fine-scale habitat differentiation
The focal fish preferentially associated with a stimulus shoal taken from near their own site of capture over the alternative (Wilcoxon test: Z=2.8, n=24, p=0.005; figure 1c). There was no difference in the strength of the shoaling preferences of focal fish from either site (Mann–Whitney U-test: U12,12=0.7, p=0.516), or in their response to the three stimulus groups (Kruskal–Wallis ANOVA: χ2,212=1.07, p=0.585).
(c) Temporal flexibility
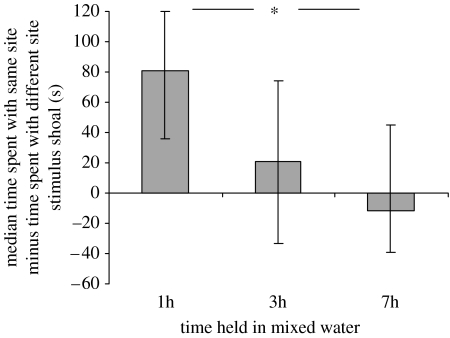
The shoaling preferences of focal fish changed over time (Kruskal–Wallis ANOVA: χ2,692=6.98, p=0.029; figure 2). Focal fish tested after being held for 1 h in mixed water preferentially associated with a stimulus shoal taken from near their own site of capture over a stimulus shoal taken from the alternative (Wilcoxon test: Z=2.6, n=24, p=0.011). Focal fish tested after 3 h in mixed water (Wilcoxon test: Z=0.9, n=24, p=0.423) and those tested after 7 h in mixed water (Wilcoxon test: Z=0.5, n=24, p=0.608) showed no significant association preference for either stimulus shoal. In each case, there was no difference between the strength of the shoaling preferences of focal fish from either site (Mann–Whitney U-tests: 1 h in mixed water: U12,12=1.2, p=0.29; 3 h: U12,12=0.39, p=0.724; 7 h: U12,12=0.44, p=0.717). In addition, there was no difference in the response of focal fish to the three stimulus groups in each case (Kruskal–Wallis ANOVAs: 1 h: χ2,212=1.23. p=0.54; 3 h: χ2,212=0.85, p=0.655; 7 h: χ2,212=0.55, p=0.761).
Figure 2.
Median time±quartiles spent by focal fish with stimulus fish from their home site where the other stimulus fish was taken from the alternative site as a function of time spent in mixed water, n=24 for each treatment. Differences in time spent shoaling by focal fish with the two stimulus shoals in binary choice tests were derived by subtracting time spent with shoal ‘b’ from time spent with shoal ‘a’ and comparing the results against a null expectation of 0. Significant differences are denoted as follows: *p<0.05.
4. Discussion
Our results show that free-ranging populations of sticklebacks use temporally flexible, local habitat-specific cues for social recognition. The focal fish preferred to associate with others that shared the same recent habitat experience as themselves. The labile association preferences observed in the current study suggest that the fish are not using learned individual recognition based on previous social experience to mediate their shoaling decisions, but are probably using self-referent matching of chemical cues. The current work suggests that rather than being based solely on simple fixed templates, social recognition in animals may be at least partially dependent on spatially and temporally flexible cues relating to their recent habitat and diet experience, allowing individuals to discriminate between subsets of their local populations.
The preference of focal fish for stimulus shoals from their own habitat over the alternative was reversed following translocation into the latter. This ability to adjust association preferences in light of recent habitat experience suggests that focal fish were self-referencing to mediate their choices rather than using recognition based on previous social experience (Hauber & Sherman 2001; Ward et al. 2005). Self-referencing, in common with a great deal of fish social recognition, is achieved through the use of chemical cues. We cannot explicitly exclude the possibility that chemical cues were augmented by visual cues; however, a number of factors suggest that this is unlikely to have been the case: we observed no differences in the behaviour or the appearance of the fish from different sampling stations (see also Webster et al. in press). Any differences that might have been exhibited would probably have been diminished by the turbidity of the water in the test tank and the shaded position in which it was situated. Similarly, we cannot exclude the possibility that fish were basing their preferences on individual recognition via previous social experience; however, the protocols that we instituted against this and the high densities of sticklebacks at the study sites would tend to militate against this as individual recognition may be a density-dependent phenomenon (see Griffiths & Magurran 1997b). Finally, individual recognition should be context independent: if sticklebacks used a learned visual recognition template, then transplanted fish would have maintained their preference for fish from their home site, instead of changing to prefer fish from their most recent habitat.
The loss of an established preference following transfer to a neutral environment was rapid, occurring within 3–4 h. This agrees with a recent laboratory study on sticklebacks (Webster et al. in press). Temporal flexibility is a pre-requisite if cues are to maintain their relevance. Previous work has shown the potential for such cues to change over time: a study on bullhead catfish (Ictalurus nebulosus) showed that when the diets of some fish were changed, recognition broke down (Bryant & Atema 1987). Similarly, studies on other fish species have shown changes in social recognition following changes of diet (Olsen et al. 2003) or water chemistry (Ward et al. 2004). In this regard, fishes show similarities to social recognition patterns in other taxa. Pfennig (1990) showed the effect of habitat cues on social recognition in spadefoot toads (Scaphiopus sp.) and Porter et al. (1989) reported similar influences on chemical signatures in spiny mice (Acomys cahirinus). Studies on invertebrates have also highlighted the role of recent environmental cues in shaping recognition cues (Linsenmair 1987; Morel & Blum 1988).
Choosing fish were able to discriminate between stimulus fish taken from sites along a continuous channel separated by 200 m, preferring to associate with those captured nearest to their own local site. This high degree of spatial resolution occurred in the absence of any measured differences in water chemistry and in a flowing water environment. The source of the variation in cues may lie in microhabitat differences between the sites arising from differences in water depth and levels of cover provided by overhanging vegetation. These factors are known to influence invertebrate populations and ultimately fish diet (Cooper et al. 1998; Heino et al. 2004).
How habitat experience translates into changes in cues is not yet understood. It is known that mucus (Matsumura et al. 2004), urine (Moore et al. 1994; Olsen et al. 1998) and faeces (Courtenay et al. 1997) all act as chemical cues in fish and can convey information on age, sex, kinship and dominance (Thom & Hurst 2004). Water chemistry clearly affects the odour profiles of fishes. This may act superficially, perhaps on the mucus coating or, given that freshwater fishes have to excrete large amounts of urine, which is derived from the water that they live in, the urine may carry a chemical signature that is representative of their immediate habitat. Diet is capable of rapidly, if transiently, affecting the chemical characteristics of urine and sweat in mammals, including humans (Mitchell 2001) and this may also be true for fishes. In future work, we hope to directly assess the chemical cues produced by the fish using techniques such as stable isotope analysis (Sorensen & Hobson 2005) or HPLC (e.g. von Elert & Pohnert 2000).
Taken as a whole, our findings suggest that the sticklebacks' social recognition mechanisms and association preferences are based primarily on self-referent chemical cue matching and not on individual recognition by previous social experience. While this challenges the current paradigm explaining the mechanism underlying familiarity, the ability of these cues to stabilize the local social interactions of fishes may provide a context in which more specific individual recognition could develop. The function of chemical cue matching is as yet unclear, but it may allow individuals to orient within their social environment and may facilitate the acquisition of relevant and current local public information (Valone & Templeton 2002) from sympatric individuals. Support for this hypothesis is provided by Webster et al.'s (in press) finding that fish which share the same recent habitat experience form more cohesive shoals, which are considered to improve their anti-predator function (Chivers et al. 1995) and foraging ability (Ward & Hart 2005). Conversely, associating with individuals that share a niche preference may elevate levels of competition (Bolnick et al. 2003), thus further work is required before firm conclusions may be drawn on the functional explanations for the observed social recognition mechanism.
Matching habitat- and diet-based cues may enable fishes to navigate within a variable social environment. The possibility also exists that such social navigation may act in concert with the ability of some fishes to orient using direct cues from the physical environment. Both trout (Salmo trutta; Halvorsen & Stabell 1990) and larval coral reef fishes (Atema et al. 2002; Døving et al. 2006) are known to navigate using localized habitat-specific chemical cues. The possibility also exists that the mechanisms of social and habitat navigation may overlap as, in some cases, ‘habitat-specific’ odour cues are actually generated by substrate marking by fish faeces (Stabell 1987; Courtenay et al. 1997; Døving et al. 2006).
The importance of chemical cues to the behaviour of aquatic animals has been demonstrated in contexts as diverse as mate choice and reproduction (Milinski et al. 2005; Miranda et al. 2005; Sorensen et al. 2005), predator–prey relationships (Jordao & Volpato 2000; Wisenden 2000; Mirza & Chivers 2001) and shoaling behaviour (Brown & Smith 1994; Behrmann-Godel et al. 2006). The challenge remains to take these studies out and to test them in the natural environment. While the present study attempts to provide biological realism by testing fish shortly after their removal from the natural environment, we remain unable to avoid manipulating the fish. Until a protocol is designed that allows the fish to remain free-ranging, useful further work would investigate both the mechanisms and the functions of this phenomenon.
Acknowledgments
This work was funded by the National Environmental Research Council (UK). The authors would like to thank Prof. Anne Magurran and three anonymous referees for their comments, which considerably improved the manuscript.
References
- Atema J, Kingsford M.J, Gerlach G. Larval reef fish could use odour for detection, retention and orientation to reefs. Mar. Ecol. Prog. Ser. 2002;241:151–160. [Google Scholar]
- Behrmann-Godel J, Gerlach G, Eckmann R. Kin and population recognition in sympatric Lake Constance perch (Perca fluviatilis L.): can assortative shoaling drive population divergence? Behav. Ecol. Sociobiol. 2006;59:461–468. doi:10.1007/s00265-005-0070-3 [Google Scholar]
- Beye M, Neumann P, Moritz R.F.A. Nestmate recognition and the genetic gestalt in the mound-building ant, Formica polyctena. Insect. Soc. 1997;44:49–58. doi:10.1007/s000400050022 [Google Scholar]
- Beye M, Neumann P, Chapuisat M, Pamilo P, Moritz R.F.A. Nestmate recognition and the genetic relatedness of nests in the ant Formica pratensis. Behav. Ecol. Sociobiol. 1998;43:67–72. doi:10.1007/s002650050467 [Google Scholar]
- Bolnick D.I, Svanback R, Fordyce J.A, Yang L.H, Davis J.M, Hulsey C.D, Forister M.L. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 2003;161:1–28. doi: 10.1086/343878. doi:10.1086/343878 [DOI] [PubMed] [Google Scholar]
- Brown G.E, Smith R.J.F. Fathead minnows use chemical cues to discriminate natural shoalmates from unfamiliar conspecifics. J. Chem. Ecol. 1994;20:3051–3061. doi: 10.1007/BF02033710. doi:10.1007/BF02033710 [DOI] [PubMed] [Google Scholar]
- Brown R.E, Schellink H.M, West A.M. The influence of dietary and genetic cues on the ability of rats to discriminate between the urinary odors of MHC congenic mice. Physiol. Behav. 1996;60:365–372. doi:10.1016/0031-9384(96)00030-3 [PubMed] [Google Scholar]
- Bryant B.P, Atema J. Diet manipulation affects social-behavior of catfish—importance of body odor. J. Chem. Ecol. 1987;13:645–1661. doi: 10.1007/BF00980206. doi:10.1007/BF00980206 [DOI] [PubMed] [Google Scholar]
- Chivers D.P, Brown G.E, Smith R.J.F. Familiarity and shoal cohesion in fathead minnows (Pimephales promelas)—implications for antipredator behavior. Can. J. Zool. 1995;73:955–960. [Google Scholar]
- Cooper S.D, Diehl S, Kratz K, Sarnelle O. Implications of scale for patterns and processes in stream ecology. Aust. J. Ecol. 1998;23:27–40. doi:10.1111/j.1442-9993.1998.tb00703.x [Google Scholar]
- Courtenay S.C, Quinn T.P, Dupuis H.M.C, Groot C, Larkin P.A. Factors affecting the recognition of population-specific odours by juvenile coho salmon. J. Fish Biol. 1997;50:1042–1060. [Google Scholar]
- Crosland M.W.J. Kin recognition in the ant, Rhytidoponera confusa. 1. Environmental odor. Anim. Behav. 1989;37:912–919. doi:10.1016/0003-3472(89)90135-8 [Google Scholar]
- Douglas R.H, Hawryshyn C.W. Behavioural studies of fish vision: an analysis of their capabilities. In: Douglas R.H, Djamgoz M.B.A, editors. The visual system of fish. Chapman and Hall; London, UK: 1990. pp. 373–418. [Google Scholar]
- Døving K.B, Stabell O.B, Ostlund-Nilsson S, Fisher R. Site fidelity and homing in tropical coral reef cardinalfish: are they using olfactory cues? Chem. Senses. 2006;31:265–272. doi: 10.1093/chemse/bjj028. doi:10.1093/chemse/bjj028 [DOI] [PubMed] [Google Scholar]
- Griffiths S.W, Magurran A.E. Familiarity in schooling fish: how long does it take to acquire? Anim. Behav. 1997a;53:945–949. doi:10.1006/anbe.1996.0315 [Google Scholar]
- Griffiths S.W, Magurran A.E. Schooling preferences for familiar fish vary with group size in a wild guppy population. Proc. R. Soc. B. 1997b;264:547–551. doi:10.1098/rspb.1997.0078 [Google Scholar]
- Griffiths S.W, Ward A.J.W. Learned recognition of conspecifics. In: Brown C, Laland K, Krause J, editors. Fish learning & behaviour. Blackwell Publishing Ltd; Oxford, UK: 2006. [Google Scholar]
- Halvorsen M, Stabell O.B. Homing behavior of displaced stream-dwelling brown trout. Anim. Behav. 1990;39:1089–1097. doi:10.1016/S0003-3472(05)80781-X [Google Scholar]
- Hauber M.E, Sherman P.W. Self-referent phenotype matching: theoretical considerations and empirical evidence. Trends Neurosci. 2001;24:609–616. doi: 10.1016/s0166-2236(00)01916-0. doi:10.1016/S0166-2236(00)01916-0 [DOI] [PubMed] [Google Scholar]
- Heino J, Louhi P, Muotka T. Identifying the scales of variability in stream macroinvertebrate abundance, functional composition and assemblage structure. Freshw. Biol. 2004;49:1230–1239. doi:10.1111/j.1365-2427.2004.01259.x [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Jordao L.C, Volpato G.L. Chemical transfer of warning information in non-injured fish. Behaviour. 2000;137:681–690. [Google Scholar]
- Krause J, Ruxton G.D. Oxford University Press; Oxford, UK: 2002. Living in groups. [Google Scholar]
- Liang D, Silverman J. “You are what you eat”: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften. 2000;87:412–416. doi: 10.1007/s001140050752. doi:10.1007/s001140050752 [DOI] [PubMed] [Google Scholar]
- Liebert A.E, Starks P.T. The action component of recognition systems: a focus on the response. Ann. Zool. Fenn. 2004;41:747–764. [Google Scholar]
- Linsenmair K.E. Kin recognition in subsocial arthropods, in particular in the desert isopod Hemilepistus reaumuri. In: Fletcher D.J.C, Michener C.D, editors. Kin recognition in animals. Wiley; Toronto, Canada: 1987. pp. 121–208. [Google Scholar]
- Mateo J.M. Recognition systems and biological organisation: the perception component of social recognition. Ann. Zool. Fenn. 2004;41:729–745. [Google Scholar]
- Matsumura K, Matsunaga S, Fusetani N. Possible involvement of phosphatidylcholine in school recognition in the catfish, Plotosus lineatus. Zool. Sci. 2004;21:257–264. doi: 10.2108/zsj.21.257. doi:10.2108/zsj.21.257 [DOI] [PubMed] [Google Scholar]
- Milinski M, Griffiths S, Wegner K.M, Reusch T.B.H, Haas-Assenbaum A, Boehm T. Mate choice decisions of stickleback females predictably modified by MHC peptide ligands. Proc. Natl Acad. Sci. USA. 2005;102:4414–4418. doi: 10.1073/pnas.0408264102. doi:10.1073/pnas.0408264102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Almeida O.G, Hubbard P.C, Barata E.N, Canario A.V.M. Olfactory discrimination of female reproductive status by male tilapia (Oreochromis mossambicus) J. Exp. Biol. 2005;208:2037–2043. doi: 10.1242/jeb.01584. doi:10.1242/jeb.01584 [DOI] [PubMed] [Google Scholar]
- Mirza R.S, Chivers D.P. Chemical alarm signals enhance survival of brook charr (Salvelinus fontinalis) during encounters with predatory chain pickerel (Esox niger) Ethology. 2001;107:989–1005. doi:10.1046/j.1439-0310.2001.00729.x [Google Scholar]
- Mitchell S.C. Food idiosyncrasies: beetroot and asparagus. Drug Metab. Dispos. 2001;29:539–543. [PubMed] [Google Scholar]
- Moore A, Ives M.J, Kell L.T. The role of urine in sibling recognition in Atlantic salmon Salmo salar (L.) parr. Proc. R. Soc. B. 1994;255:173–180. doi:10.1098/rspb.1994.0025 [Google Scholar]
- Morel L, Blum M.S. Nestmate recognition in Camponotus floridanus callow worker ants: are sisters or nestmates recognized? Anim. Behav. 1988;36:718–725. doi:10.1016/S0003-3472(88)80154-4 [Google Scholar]
- Olsen K.H, Grahn M, Lohm J, Langefors A. MHC and kin discrimination in juvenile Arctic charr, Salvelinus alpinus (L.) Anim. Behav. 1998;56:319–327. doi: 10.1006/anbe.1998.0837. doi:10.1006/anbe.1998.0837 [DOI] [PubMed] [Google Scholar]
- Olsen K.H, Grahn M, Lohm J. The influence of dominance and diet on individual odours in MHC identical juvenile Arctic charr siblings. J. Fish Biol. 2003;63:855–862. doi:10.1046/j.1095-8649.2003.00185.x [Google Scholar]
- Pfennig D.W. “Kin recognition” among spadefoot toad tadpoles: a side effect of habitat selection? Evolution. 1990;44:785–798. doi: 10.1111/j.1558-5646.1990.tb03805.x. doi:10.2307/2409546 [DOI] [PubMed] [Google Scholar]
- Porter R.H, McFadyen-Ketchum S.A, King G.A. Underlying bases of recognition signatures in spiny mice Acomys cahirinus. Anim. Behav. 1989;37:638–644. doi:10.1016/0003-3472(89)90042-0 [Google Scholar]
- Rice W.R. Analysing table of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. doi:10.2307/2409177 [DOI] [PubMed] [Google Scholar]
- Schellink H.M, Slotnick B.M, Brown R.E. Odors of individuality originating from the major histocompatibility complex are masked by diet cues in the urine of rats. Anim. Learn. Behav. 1997;25:193–199. [Google Scholar]
- Sorensen P.W, Hobson K.A. Stable isotope analysis of amphidromous Hawaiian gobies suggests their larvae spend a substantial period of time in freshwater river plumes. Environ. Biol. Fishes. 2005;74:31–42. doi:10.1007/s10641-005-3212-6 [Google Scholar]
- Sorensen P.W, Fine J.M, Dvornikovs V, Jeffrey C.S, Shao F, Wang J.Z, Vrieze L.A, Anderson K.R, Hoye T.R. Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat. Chem. Biol. 2005;1:324–328. doi: 10.1038/nchembio739. doi:10.1038/nchembio739 [DOI] [PubMed] [Google Scholar]
- Stabell O.B. Intraspecific pheromone discrimination and substrate marking by Atlantic salmon parr. J. Chem. Ecol. 1987;13:1625–1643. doi: 10.1007/BF00980205. doi:10.1007/BF00980205 [DOI] [PubMed] [Google Scholar]
- Thom M.D, Hurst J.L. Individual recognition by scent. Ann. Zool. Fenn. 2004;41:765–787. [Google Scholar]
- Todd J.H, Atema J, Bardach J.E. Chemical communication in social behaviour of a fish, the yellow bullhead (Ictalarus natalis) Science. 1967;158:672–673. doi: 10.1126/science.158.3801.672. doi:10.1126/science.158.3801.672 [DOI] [PubMed] [Google Scholar]
- Valone T.J, Templeton J.J. Public information for the assessment of quality: a widespread social phenomenon. Phil. Trans. R. Soc. B. 2002;357:1549–1557. doi: 10.1098/rstb.2002.1064. doi:10.1098/rstb.2002.1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elert E, Pohnert G. Predator specificity of kairomones in diel vertical migration of Daphnia: a chemical approach. Oikos. 2000;88:119–128. doi:10.1034/j.1600-0706.2000.880114.x [Google Scholar]
- Ward A.J.W, Hart P.J.B. Foraging benefits of shoaling with familiars may be exploited by outsiders. Anim. Behav. 2005;69:329–335. doi:10.1016/j.anbehav.2004.06.005 [Google Scholar]
- Ward A.J.W, Krause J. Body length assortative shoaling in the European minnow, Phoxinus phoxinus. Anim. Behav. 2001;62:617–621. doi:10.1006/anbe.2001.1785 [Google Scholar]
- Ward A.J.W, Botham M.S, Hoare D.J, James R, Broom M, Godin J.-G.J, Krause J. Association patterns and shoal fidelity in the three-spined stickleback. Proc. R. Soc. B. 2002;269:2451–2455. doi: 10.1098/rspb.2002.2169. doi:10.1098/rspb.2002.2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A.J.W, Hart P.J.B, Krause J. The effects of habitat- and diet-based cues on association preferences in three-spined sticklebacks. Behav. Ecol. 2004;15:925–929. doi:10.1093/beheco/arh097 [Google Scholar]
- Ward A.J.W, Holbrook R.I, Krause J, Hart P.J.B. Social recognition in sticklebacks: the role of direct experience and habitat cues. Behav. Ecol. Sociobiol. 2005;57:575–583. doi:10.1007/s00265-004-0901-7 [Google Scholar]
- Webster, M. M., Goldsmith, J., Ward, A. J. W. & Hart, P. J. B. In press. Habitat specific chemical cues influence association preferences and shoal cohesion in fish. Behav. Ecol. Sociobiol.
- Wisenden B.D. Olfactory assessment of predation risk in the aquatic environment. Phil. Trans. R. Soc. B. 2000;355:1205–1208. doi: 10.1098/rstb.2000.0668. doi:10.1098/rstb.2000.0668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt T.D. Cambridge University Press; Cambridge, UK: 2003. Pheromones and animal behaviour. Communication by smell and taste. [Google Scholar]