Abstract
Our newly developed underwater high definition video camera system took the first live images of adults of the mesopelagic large squid, Taningia danae, between 240 and 940 m deep off Ogasawara Islands, western North Pacific. The resulting footage includes attacking and bioluminescence behaviours, and reveals that T. danae is far from the sluggish neutrally buoyant deep-sea squid previously suspected. It can actively swim both forward and backward freely by flapping its large muscular triangular fins and changes direction quickly through bending its flexible body. It can attain speeds of 2–2.5 m s−1 (7.2–9 km h−1) when attacking bait rigs. They emitted short bright light flashes from their large arm-tip photophores before final assault, which might act as a blinding flash for prey as well as a means of measuring target distance in a dark deep-sea environment. They also emitted long and short glows separated by intervals while wandering around the double torch lights attached to the bait rig, suggestive of potential courtship behaviours during mating.
Keywords: large deep-sea squid, underwater high definition video camera system, Ogasawara Islands, swimming ability, attacking behaviours, bioluminescence
1. Introduction
The deep-sea eight-armed squid Taningia danae, Joubin 1931, is recognized as one of the largest mesopelagic squids in tropical and subtropical oceans worldwide (Clarke 1966; Nesis 1982; Roper & Vecchione 1993). The largest previously reported is a mature female of 2.3 m total length and weighing 61.4 kg, which was caught by bottom trawl near Georges Basin in the North Atlantic (Roper & Vecchione 1993).
Taningia danae is characterized by having a heavy conical mantle with large thick triangular fins, eight relatively short arms with biserial strong hooks, and the loss of feeding tentacles in adults. Additionally, it has extremely large oval photophores on the distal end of dorsolateral arms. The photophores are cream-white in colour and usually covered by thin dark epidermis lids. Roper & Vecchione (1993) observed bioluminescence of a young individual in a shipboard aquarium and reported that when it was stimulated, bright flashes of brilliant blue–green light emitted simultaneously from both arm-tip photophores.
Small juvenile individuals of T. danae have been collected with a variety of biological mid-water nets (Clarke & Lu 1974; Okutani 1974; Lu & Clarke 1975a,b), however, large mature individuals have rarely been captured by fishing gear. Most records of adult T. danae have come from the stomach contents of sperm whales (Clarke 1967; Okutani & Satake 1978). Several hundred large beaks (mandibles) of T. danae were found in sperm whale stomachs (Clarke 1980; Clarke & MacLeod 1982). These studies suggested that T. danae is a very abundant deep-sea large squid in the tropical and subtropical oceans of the world. However, no previous study had observed a live T. danae in its natural environment.
During our research on the biomass and composition of large meso- and bathypelagic cephalopods of Japanese waters (Kubodera & Mori 2005), the first live image was obtained of T. danae individuals in the deep waters off Chichijima Islands (ca 26–27° N, 142° E) in the North Pacific in 2005 with newly developed underwater high definition video camera system. The resulting footage shows us remarkable hunting behaviour of T. danae in its natural environment, as well as, bioluminescence from the large arm-tip photophores.
2. Material and methods
(a) Underwater high definition video camera system
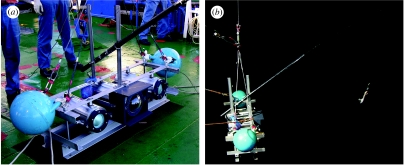
The system consisted of three hi-stainless steel cylindrical housings, measuring 21.6 cm in diameter and 50 cm in length, and a stainless steel frame (130×50×45 cm) for mounting the housings horizontally (figure 1a). The housings were designed to withstand pressures to 1500 m deep. A high definition video camera (SONY-FX1) with a fish-eye lens was set in the central housing, which had a dome-shaped front glass to cover nearly 180° angle of the fish-eye lens. A halogen light (24 V×150 W) with rechargeable battery was set in both right and left housings. Camera and lights were controlled on and off with separate built-in timers. Total system weight was approximately 170 kg in air. Two large floats were attached to the frame for reducing its weight in water. The system was designed and developed under our consultation by Goto Aquatics (http://www.goto-aqua.co.jp).
Figure 1.
(a) Underwater high definition video camera system and (b) attached pole with bait rig.
(b) Bait rig and filters
A 2.2 m glass fibre pole was attached obliquely from the frame. A bait rig was suspended from the tip of the pole with a 1.5 m nylon monofilament line, weighted with a pencil-shaped underwater torch (ca 15 cm). At the end, a side branch line of 18–20 cm length was attached that bore a single hook with a fresh Japanese common squid (Todarodes pacificus) of 22–25 cm mantle length and/or fresh mackerel of 25–30 cm total length (figure 1b). A mesh bag filled with freshly mashed euphausiid shrimps was attached to the frame as an odour lure. Length of the bait rig and position at which bait rig was suspended from the pole were adjusted to obtain the best images. One or two underwater torches were used in each deployment. Blue and/or red filters were attached to the main halogen lights on some deployments.
(c) Configuration of timers
A mini DV video tape could record only 1 h of footage when the camera is operating continuously. We configured built-in timers on the camera and the lights to work as 5 min on and 5 min off. Usually, the camera was set to start 10 s before the lights turn on. This made the system record nearly 2 h of observation without requiring tape change.
(d) Ship board operation
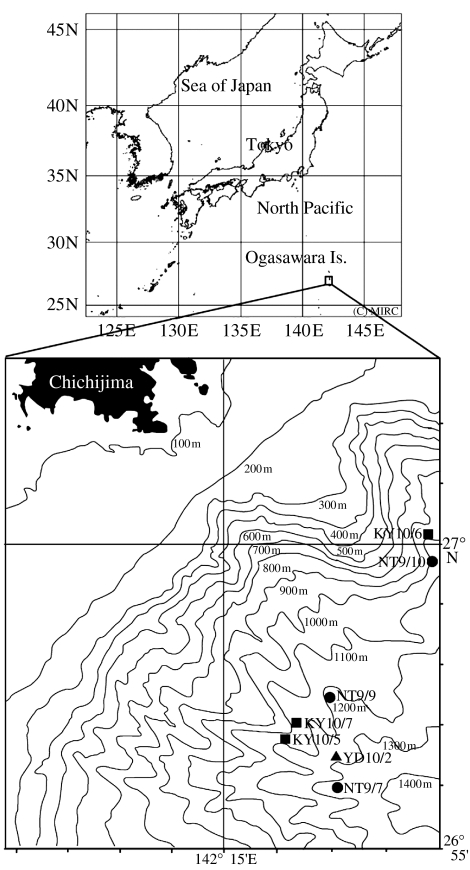
Three vessels, the R/V Natsushima (1739 tonnage: Japan Agency for Marine-Earth Science and Technology), the R/V Koyo (46 tonnage: Ogasawara Fisheries Centre) and the F/V Yudai-Maru (10 tonnage: Captain Y. Hirayama) were available for this research. The camera system was suspended in mid-water at designated depths of 800–950 m for 1–2 h and occasionally recovered with stops at intermediate depths for 30–40 min before retrieval. A digital depth and water temperature recorder was attached to the system for monitoring the depth at which footage was taken. Eight deployments were made on the R/V Natsushima once per day in the evening between the 7 and 14 September, 2005, and 16 deployments were conducted from the R/V Koyo and two from the F/V Yudai-Maru between the 1 and 7 October, 2005 in the waters about 10–15 miles southeastern off Chichijima Island.
3. Results
A total of 26 deployments were made, of which T. danae was observed during 12 (figure 2). Time, depth and duration of occurrence, action observed, distance from the system, filter colour, number of torches, bait materials, flash duration of arm-tip photophores and remarks are summarized in table 1 in the electronic supplementary material. Video clips 1–5 (Quick time movies, 0.9–5.7 MB) are also provided in the electronic supplementary material.
Figure 2.
Stations at which Taningia danae was observed with underwater high definition video camera system in 2005. NT: R/V Natsushima, YD: F/V Youdai-Maru and R/V KY: Koyo, month/day.
(a) Vertical distribution
Taningia danae were caught on video at depths ranging from 240 to 940 m. They occurred at 614 and 940 m depth in the morning (08.00–09.00) and at 900 m in the late afternoon (16.00). Around sunset (17.00–19.00), they occurred at depths ranging from 480 to 800 m, mainly at 500–600 m. After sunset (20.00–23.00), they occurred at depth of between 240 and 650 m.
(b) Swimming behaviour
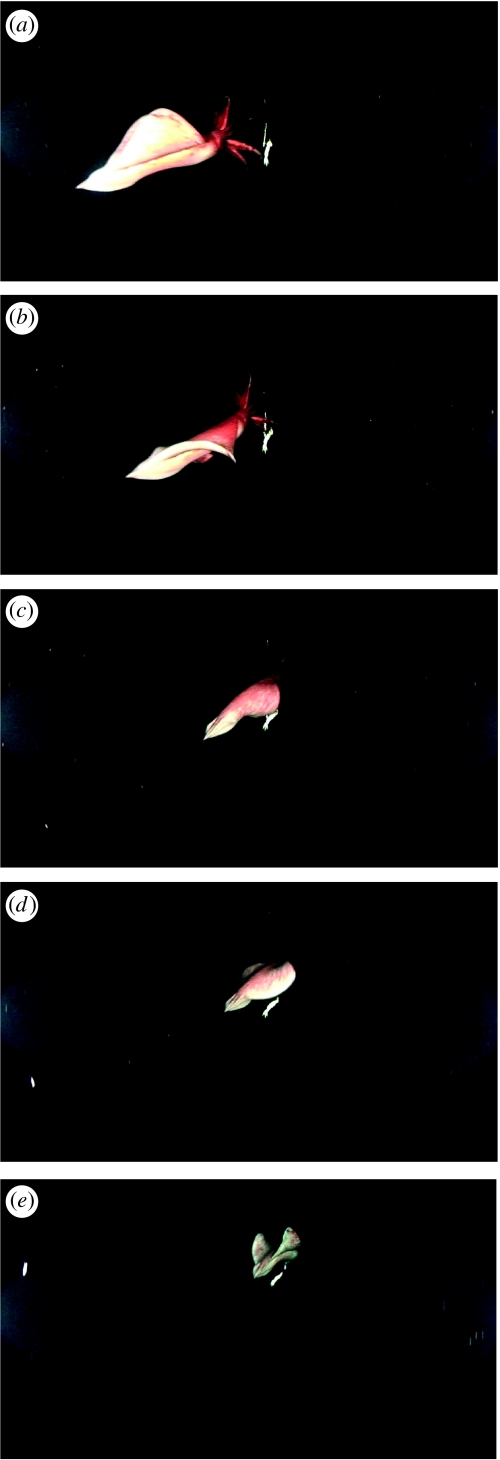
Taningia danae swam both forward (head and arms forefront) and backward (fin tip forefront) and rapidly changed direction (video clip 1 in electronic supplementary material). Both movements were primarily generated by flapping the large and muscular triangular fins. While swimming in either direction, arms were held together compactly. Video clip 2 in electronic supplementary material shows the quick motion of attacking the rig line straight forward extending all arms, then somersaulting quickly (out of camera frame) and returning (backward) by wrapping the entire fins ventrally. When swimming forward, a cycle of fin movement was as follows: (i) holding up fins dorsally; (ii) moving down anterior rim of fins; (iii) followed by downward movement from lateral to posterior portions of fins; (iv) wrapping whole fins ventrally; (v) moving up anterior rim of fins; (vi) followed by upward movement of lateral and posterior portions of fins; and (vii) holding up fins dorsally (figure 3). No pumping of mantle was observed during the cycle. Lithe downward and upward movements from lateral to posterior portion of fins generated powerful pause-less forward propulsion. In this case, the cycle took just 1 s. Judging from the distance between camera and bait squid (ca 1.6–1.8 m), and bait squid size (ca 30 cm total length), it moved nearly 2.0–2.5 m in a cycle, hence, it attained a swimming speed of 2–2.5 m s−1 (7.2–9 km h−1). By comparison with the size of the bait squid size (figure 3e), this individual is more than 1 m in total length.
Figure 3.
A cycle of fin movements in forward swimming: (a) holding up fins dorsally; (b) moving down anterior edge of fins, downward movement from lateral to posterior portions of fins; (c) wrapping whole fins ventrally; (d) moving up anterior border of fins, upward movement of lateral and posterior portions of fins and (e) holding up fins dorsally.
(c) Attacking behaviour
Fourteen attacking behaviours were observed, of which five were directly towards the bait and/or torch, four to the rig line, three to the halogen light and the other two attacked the pole tip.
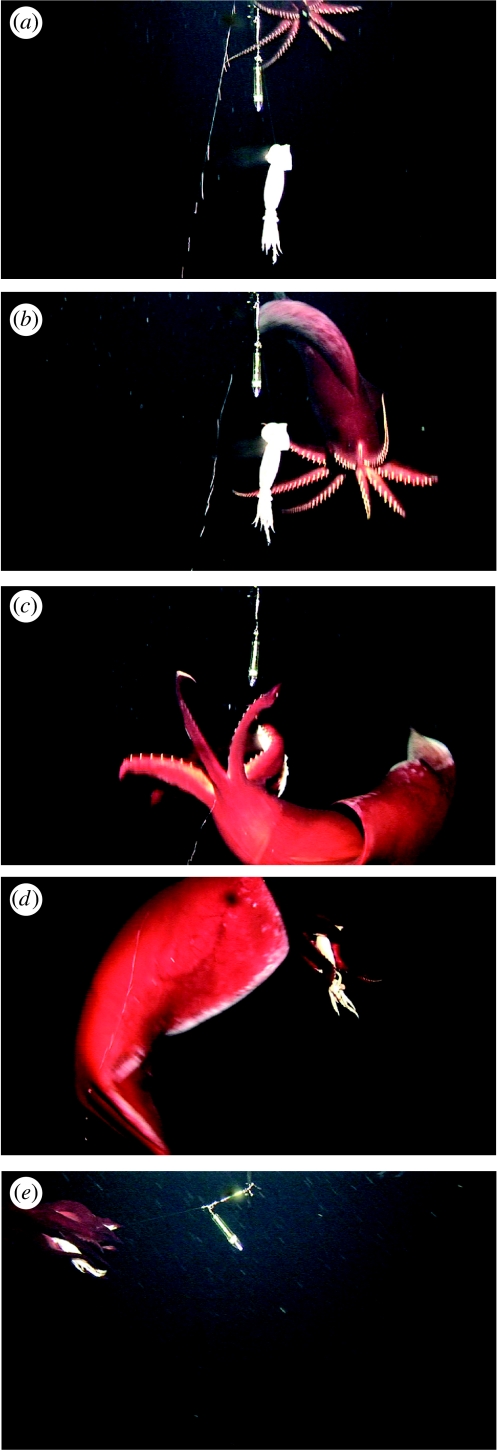
Bait and/or torch attacking was observed twice in white light conditions and twice in blue light. None was observed under red light conditions. This attacking behaviour was associated with: (i) swimming forward to approach the bait; (ii) spreading all arms widely, occasionally emitting a short flash from arm-tip photophores (1.2–1.6 s; three times out of four observations); (iii) turning round the bait twisting the body to catch the bait with the ventral arms; (iv) turning the body flexibly to hold the whole bait with all arms; and (v) swimming away backward, detaching the bait from the hook (figure 4). Video clip 3 in electronic supplementary material shows short flash emitting (1.2 s) just before reaching to the bait.
Figure 4.
Bait attacking behaviour: (a) swimming forward to approach toward the bait; (b) spreading all arms widely; (c) swimming round the bait, twisting the body to touch the bait with ventral arms; (d) turning the body and (e) holding whole bait with all arms, swimming away backward.
Rig line attacking was observed twice in white light, once in red light and once in blue light conditions. This behaviour differed from above bait and/or torch attacking by: (i) swimming forward to approach middle to lower portions of rig line; (ii) occasionally emitting a relatively long flash (2.1–2.2 s; twice out of four observations) before extending the arms; (iii) attacking at the rig line front on without turning round nor twisting the body; and (iv) failure to hold the line (video clip 2 in electronic supplementary material).
Halogen light attacking was observed three times in blue light conditions. This behaviour was associated with: (i) swimming forward; (ii) extending all arms; (iii) occasionally swimming round the bait (once out of three observations); (iv) banging hard at the light straight forward; and (v) swimming away backwards (video clip 4 in electronic supplementary material).
Pole tip attacking was observed twice in white light conditions. This attacking behaviour was analogous with the bait and/or torch attacking behaviour.
(d) Bioluminescence
Besides a short flash of arm-tip photophores during attacking behaviours, long glows (4.4–8.5 s) of arm-tip photophores was observed four times in red light conditions without an attack. This behaviour was associated with: (i) swimming forward, approaching the bait while emitting a long glow; (ii) keeping a certain distance from the bait; (iii) holding a little space between photophores; and (iv) extinguishing the light and swimming away. In other cases, long glows were recorded when the squid approached the baits and several short glows separated by intervals were recorded when the squid swam around the torch lights (video clip 5 in electronic supplementary material).
4. Discussion
As in the largest squid, Architeuthis, T. danae incorporates numerous tiny vacuoles of ammonia solution within its flesh to enable neutral buoyancy (Clarke et al. 1979). This system makes body musculature flabby and soft to touch in captured animals, leading a number of authors to propose that large ammonical squids are likely to be sluggish and relatively inactive (Hanlon & Messenger 1966; Roper & Boss 1982; Norman 2000; Nixon & Young 2003). Recent observations of live giant squid in the wild (Kubodera & Mori 2005) revealed that this species is much more active predator than previously suggested. Our in situ observations also show that T. danae is an aggressive and tenacious predator rather than a sluggish, inactive squid.
(a) Vertical distribution
Ontogenetic descent from the surface to depths of 200–300 m by juvenile and young T. danae (6–15 mm ML) has been reported (Roper & Vecchione 1993). Adults have been suggested to live at depths of over 1000 m based on the foraging depths of their main predators, sperm whales and deep-sea sharks (Clarke & Merrett 1972; Clarke 1977). Direct evidence of adult distributions was only a bottom trawl catch of a mature female at 260 m depth (Roper & Vecchione 1993).
Our video recordings reveal that T. danae tend to stay in a relatively deep layer (around 600–900 m) during the day and ascend to a shallow layer (around 240–500 m) at night. Tanigia danae appears to undertake short distance diel vertical migrations, similar to other mesopelagic inhabitants (Roper & Young 1975; Watanabe et al. 1999, 2006).
(b) Swimming behaviour and ability
Active short-finned squids, such as ommastrephids and loliginids generally depend on strong water ejection through the funnel as their main form of swimming propulsion (Packard 1972). Swimming directions are controlled by turning the funnel opening in opposite directions. This jet propulsion system works to generate strong acceleration backwards but is less effective for forward movement, due to the inefficiency of bending the funnel in reverse direction (Foyle & O'Dor 1987). The swimming system of T. danae is significantly different from these short-finned epipelagic squids. Vecchione et al. (2002) observed swimming behaviours of three species of deep-sea squids having large fins, among which Octopoteutihs megaptera seems to share the same swimming system as T. danae.
Taningia danae can swim forward and backwards freely by flapping large triangular fins, as well as rapidly changing direction by bending its flexible body. Fin movement of T. danae, used to generate forward propulsion, is similar to that of rays (i.e. Family Dasyatidae), although rays can only swim forward smoothly. Tanigia danae can reverse fin undulation to generate backward propulsion. Undulation of full-length muscular fins has advantages over jet propulsion, in that it generates the same acceleration power for either direction, without the pumping pause necessary in jet propulsion. Tanigia danae of approximately 1 m total length can attain speeds of 2–2.5 m s−1 (7.2–9 km h−1) in attacking bait rigs. No reliable information on swimming velocity of deep-sea squids is available. Yano et al. (2000) reported that the epi- to mesopelagic muscular squid, Thysanoteuthis rombus, which have similar large triangular fins and grow nearly the same size as T. danae, migrated at an average speed of 2.0 km h−1 over 3 days of biotelemetry observation. Direct comparison between aggressive swimming velocity and average migration speed cannot be made but potential swimming ability for T. danae may be comparable.
(c) Attacking and hunting behaviours
In addition to the eight arms, most common and active squids have a pair of relatively long and elastic feeding tentacles. When the prey are appropriate in size and slow movers, T. pacificus approach front on (attention stage), maintain a certain distance from the prey (positioning stage), rapidly extending the tentacles to grip the prey with suckers on tentacluar clubs (seizure stage), finally drawing the prey into the arms holding it with the arm suckers (aquarium observation filmed by Y. Sakurai). When prey are larger and faster, Illex illecebrosus usually stalk the prey and approach gradually before a sudden final forward assault, using all arms rather than the feeding tentacles to capture the prey (Foyle & O'Dor 1987).
From 14 observations, we recognized three different attacking behaviours in T. danae. Bait and/or torch attacking is considered to be a typical hunting behaviour for suitable prey. Tanigia danae lacks feeding tentacles so it rushes at the prey without positioning and uses the ventral arms as analogues of the feeding tentacles of other squids. Rig line attacking is hard to define but reflection of nylon monofilament line and/or the connecting swivel may attract or dazzle the squid. Attacking to the blue-filtered halogen light might be an aggressive behaviour against potential adversaries. Blue light seemed provocative.
(d) Bioluminescence
Many mesopelagic small to medium sized squids possess numerous small photophores on the ventral surface of the body, used for countershading, i.e. enoploteuthids and histioteuthids (Clarke 1963; Young & Roper 1976; Young 1977). Some possess relatively large photophores on the ventral surface of eyes, on the tips of the arms and/or inside the mantle cavity. The role of these photophores has been suggested to be signalling species-identity and/or sexual recognition at maturity (Hanlon & Messenger 1966. Roper & Vecchione (1993) reported two primary responses of bioluminescence from the large arm tip photophores of young T. danae on stimulation in a shipboard aquarium. The most common reaction was the coordinated flashes accompanied by an attack, grasping the researcher's fingers and biting. The second reaction involved a bright flash, followed by rapid retreat from the stimulus. These authors suggested that the flashing might serve as an offensive behaviour that disrupts defences of prey.
Our observations of wild adult T. danae recorded this first behaviour of bioluminescence accompanied by an attack. Light emission lasted 1.2–1.6 s just before turning the body to the bait and was extinguished when capturing the bait. This emission may work as a blinding flash for the prey, as well as a means of illumination and measuring target distance in an otherwise dark environment. By comparison, a relatively long emission (2.1–2.2 s) was observed during bait rig line attacking. It was not certain that this emission served the same function as prey attacking, but instead seemed to be a precautionary signal used when approaching unidentified objects. The most interesting bioluminescence observed was a long glow when approaching (4.4–8.5 s) and several short glows separated by intervals when wandering around the bait without attacking. We believe that this behaviour may represent attempts at communication with conspecifics using bioluminescence. We propose that the light given off by the double torch lights may resemble the long glows of the arm-tip photophores of T. danae and may attract them, eliciting long glows when approaching. Short glows were emitted when investigating the double-torch lights but in the absence of an appropriate response the squid moved on. This behaviour was observed only in red light conditions.
(e) Light conditions and effect of torch light
Two strong halogen lights (24 V, 150 W) were used for lighting up the dark environment, which inevitably disturbs the deep-sea environment. During the research cruise of the R/V Koyo, we placed red or blue filters on the halogen lights making it possible to compare observations in different light conditions. Red light is absorbed rapidly in the water (within 10–20 m) and blue light penetrates water the deepest (several hundreds metres: Smith 1976). Many mesopelagic squids possess eyes that are characterized by having a single visual pigment with peak absorbance at 480 nm wavelength (Seidou et al. 1990). As a result, they only have vision in a blue light spectrum.
In our observations, hunting behaviours by T. danae always occurred under white and blue light conditions. This suggested that they recognized bait squid/mackerel as suitable prey under strong white and blue light conditions and successfully hunted under such artificial lighting. In some cases, strong white light caused erroneous responses. Strong blue light may cause overexposure in their high blue sensitivity, which may explain aggressive attacks on the blue-filtered halogen light. On the other hand, faint blue light from the torch appeared to attract animals. This faint light may have been less visible under strong halogen light (white and blue) so that T. danae was unable to recognize it. It was only in the red light conditions that they appeared to interact with the torch lights, as a potential form of bioluminescent communication. The observed long and short glows separated by intervals when squid were wandering around the torch under red light may represent communication, courtship or mating behaviours.
Acknowledgments
We thank Prof. T. Ura and his team, University of Tokyo, for permitting us to do our operations during his research cruise to Ogasawara Islands in 2005; Captain F. Saito and crew of the R/V Natushima for their cooperation; Captains I. Gonoi of the R/V Koyo and Y. Hirayama of the F/V Youdai-Maru and their crew for their great efforts in conducting field surveys; Mr K. Yamaguchi, Ogasawara Fisheries Centre, for his help. Dr M. Norman, Museum Victoria and Dr C. C. Lu, National Chung Hsing University gave us invaluable comments and suggestions on preparing the manuscript. This work was partially supported by Japan Broadcasting Corporation (NHK) and copyright of all figures and video clips belong to NHK.
Supplementary Material
Summary of the observations of Tanigia danae with underwater video camera system off Ogasawara Islands in 2005
Swimming forwards and backwards
Quick motion of attacking the rig line
Short flash emitting (1.2 sec.) just before reaching to the bait
Halogen light attacking
Long glow when approaching and short glows separated by intervals around the torch lights
References
- Clarke W.D. Function of bioluminescence in mesopelagic organisms. Nature. 1963;198:1244–1246. doi:10.1038/1981244a0 [Google Scholar]
- Clarke M.R. A review of the systematics and ecology of oceanic squids. Adv. Mar. Biol. 1966;4:91–300. [Google Scholar]
- Clarke M.R. A deep-sea squid, Taningia danae Joubin, 1931. Symp. Zool. Soc. Lond. 1967;19:127–143. [Google Scholar]
- Clarke M.R. Beaks, nets and numbers. Symp. Zool. Soc. Lond. 1977;38:89–126. [Google Scholar]
- Clarke M.R. Cephalopods in the diet of sperm whales of the southern hemisphere and their bearing on sperm whale biology. Discov. Rep. 1980;37:1–324. [Google Scholar]
- Clarke M.R, Lu C.C. Vertical distribution of cephalopods at 30° N 23° W in the North Atlantic. J. Mar. Biol. Assoc. UK. 1974;54:969–984. [Google Scholar]
- Clarke M.R, MacLeod N. Cephalopod remains from the stomachs of sperm whales caught in the Tasman Sea. Mem. Natl. Mus. Victoria. 1982;43:25–42. [Google Scholar]
- Clarke M.R, Merrett N.R. The significance of squid, whale and other remains from the stomachs of bottom-living deep sea fish. J. Mar. Biol. Assoc. UK. 1972;52:599–603. [Google Scholar]
- Clarke N.R, Denton R.J, Gilpin-Brown J.B. On the use of ammonium for buoyancy in squids. J. Mar. Biol. Assoc. UK. 1979;59:259–276. [Google Scholar]
- Foyle T.P, O'Dor R.K. Predatory strategies of squid (Illex illecebrousus) attacking small and large fish. Mar. Behav. Physiol. 1987;13:155–168. [Google Scholar]
- Hanlon R.T, Messenger J.B. Cephalopod behaviour. Cambridge University Press; Cambridge, UK: 1996. pp. 1–232. [Google Scholar]
- Lu C.C, Clarke M.R. Vertical distribution of cephalopods at 40° N, 53° N and 60° N at 20 W in the North Atlantic. J. Mar. Biol. Assoc. UK. 1975a;55:143–163. [Google Scholar]
- Lu C.C, Clarke M.R. Vertical distribution of cephalopods at 11° N 20° W in the North Atlantic. J. Mar. Biol. Assoc. UK. 1975b;55:369–389. [Google Scholar]
- Kubodera T, Mori K. First-ever observations of a live giant squid in the wild. Proc. R. Soc. B. 2005;272:2583–2586. doi: 10.1098/rspb.2005.3158. doi:10.1098/rspb.2005.3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesis K.N. Cephalopods of the world. Squids, cuttlefishes, octopuses, and their allies. TFH Publications; Neptune City, NJ: 1982. [Google Scholar]
- Nixon M, Young J.Z. Oxford University Press; Oxford, UK: 2003. The brains and lives of cephalopods. pp. 1–392. [Google Scholar]
- Norman M. Conch Books; Hackenheim, Germany: 2000. Cephalopods a world guide. pp. 1–320. [Google Scholar]
- Okutani T. Epipelagic decapod cephalopods collected by micronekton tows during the EASTROPAC Expedition, 1967–1968. (Systematic part) Bull. Tokai Reg. Fish. Res. Lab. 1974;80:29–118. [Google Scholar]
- Okutani T, Satake Y. Squids in the diet of 38 sperm whales caught in the Pacific off northern Honshu, Japan, February 1977. Bull. Tokai Reg. Fish. Res. Lab. 1978;93:13–27. [Google Scholar]
- Packard A. Cephalopods and fish: the limits of convergence. Biol. Rev. 1972;47:241–307. doi:10.1086/407285 [Google Scholar]
- Roper C.F.E, Boss K.J. The giant squid. Sci. Am. 1982;246:96–105. [Google Scholar]
- Roper C.F.E, Vecchione M. A geographic and taxonomic review of Taningia danae Joubin, 1931 (Cephalopoda: Octopoteuthidae), with new records and observations on bioluminescence. In: Okutani T, O'Dor R.K, Kubodera T, editors. Recent advances in fisheries biology. Tokai University Press; Kanagawa, Japan: 1993. pp. 441–456. [Google Scholar]
- Roper C.F.E, Young R.E. Vertical distribution of pelagic cephalopods. Smithson. Contrib Zool. 1975;209:1–51. [Google Scholar]
- Seidou M, Sugahara M, Uchiyama H, Hiraki K, Hamanaka T, Michinomae M, Yoshihara K, Kito Y. On the three visual pigmentsin the retina of the firefly squid, Watasenia scintillans. J. Comp. Physiol. A. 1990;166:769–773. doi:10.1007/BF00187321 [Google Scholar]
- Smith R.L. Part I-2. Waters of the sea: the ocean's characteristics and circulation. In: Cushing D.H, Walsh J.J, editors. The ecology of the sea. Blackwell; Oxford, UK: 1976. pp. 23–58. [Google Scholar]
- Vecchione M, Roper C.F.E, Widder E.A, Frank T.M. In-situ observations on three species of large-finned deep-sea squids. Bull. Mar. Sci. 2002;71:893–901. [Google Scholar]
- Watanabe H, Moku M, Kawaguchi K, Ishimaru K, Ohono A. Diel vertical migration of myctophid fishes (family Myctophidae) in the transitional waters of the western North Pacific. Fish. Oceanogr. 1999;8:115–127. doi:10.1046/j.1365-2419.1999.00103.x [Google Scholar]
- Watanabe H, Kubodera T, Moku M, Kawaguch K. Diel vertical migration of squid in the warm core ring and cold water masses in the transition region of the western North Pacific. Mar. Ecol. Prog. Ser. 2006;315:187–197. [Google Scholar]
- Yano K, Ochi Y, Shimizu H, Kasuge T. Diurnal swimming patterns of the diamond back squid as observed by ultrasonic telemetry. In: Elier J.H, Alcom D.J, Neuman M.R, editors. Biotelemetry 15: Proceedings of the 15th International Symposium on Biotelemetry, Juneau, Alaska 9–14 May 1999. International Society on Biotelemetry; Wageningen, The Netherlands: 2000. pp. 108–116. [Google Scholar]
- Young R.E. Ventral bioluminescent countershading in midwater cephalopods. Symp. Zool. Soc. Lond. 1977;38:161–190. [Google Scholar]
- Young R.E, Roper C.F.E. Bioluminescent countershading in midwater animals: evidence from living squid. Science. 1976;191:1046–1048. doi: 10.1126/science.1251214. doi:10.1126/science.1251214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of the observations of Tanigia danae with underwater video camera system off Ogasawara Islands in 2005
Swimming forwards and backwards
Quick motion of attacking the rig line
Short flash emitting (1.2 sec.) just before reaching to the bait
Halogen light attacking
Long glow when approaching and short glows separated by intervals around the torch lights