Abstract
Extensive research over the last few decades has revealed that many acoustically communicating animals compensate for the masking effect of background noise by changing the structure of their signals. Familiar examples include birds using acoustic properties that enhance the transmission of vocalizations in noisy habitats. Here, we show that the effects of background noise on communication signals are not limited to the acoustic modality, and that visual noise from windblown vegetation has an equally important influence on the production of dynamic visual displays. We found that two species of Puerto Rican lizard, Anolis cristatellus and A. gundlachi, increase the speed of body movements used in territorial signalling to apparently improve communication in visually ‘noisy’ environments of rapidly moving vegetation. This is the first evidence that animals change how they produce dynamic visual signals when communicating in noisy motion habitats. Taken together with previous work on acoustic communication, our results show that animals with very different sensory ecologies can face similar environmental constraints and adopt remarkably similar strategies to overcome these constraints.
Keywords: animal communication, background noise, signal detection, territorial displays, Anolis
1. Introduction
Background noise from wind and other sources constrains the distance over which acoustic signals can be discriminated by animals (Wiley & Richards 1982; Ryan & Brenowitz 1985; Langemann et al. 1998; Lengagne et al. 1999; Aubin & Jouventin 2002; Lengagne & Slater 2002; Lohr et al. 2003). Numerous studies document how animals counter this masking effect by tailoring acoustic properties to enhance the transmission of vocalizations produced in ‘noisy’ habitats (reviewed in Brumm & Slabbekoorn 2005 and Patricelli & Blickley 2006). Examples are taxonomically diverse and include amphibians (e.g. Ryan et al. 1990; Witte et al. 2005; Feng et al. 2006), birds (e.g. Slabbekoorn & Smith 2002; Pytte et al. 2003; Slabbekoorn & Peet 2003; Leonard & Horn 2005) and mammals (e.g. Rabin et al. 2003; Brumm et al. 2004; including humans, e.g. Lane & Tranel 1971). However, very little is known about the influence of background noise on animal communication outside the acoustic modality. Visual noise from the movement of windblown vegetation is predicted to limit the detection of movement-based visual displays (Fleishman 1992; Peters & Evans 2003a,b). Yet, no study has actually investigated whether animals change how they produce dynamic visual displays when communicating in noisy motion environments. We do so here for two species of visually communicating lizard on the island of Puerto Rico.
Anole lizards (Anolis spp.) communicate using vertical movements of the head, known as head-bobs, and an expandable throat fan or dewlap. These displays are critical for males in the maintenance of territories that overlap those of several females (Stamps 1983). Most display activity occurs on elevated perch sites inside a male's territory and serves a similar purpose to the long-range broadcast calls of birds that advertise continued territory occupancy and deter territorial intrusions (Jenssen 1977; Fox et al. 2003). As anole displays are essential for territorial defence and because broadcast signals are particularly susceptible to environmental degradation (Wiley & Richards 1982; Naguib & Wiley 2001), anole displays should be under considerable selective pressure to remain conspicuous when conditions for communication are less than ideal.
Adult male Anolis cristatellus and A. gundlachi are small (snout-to-vent length in cm, mean±s.e.m: n=12, 6.3±0.16 and n=21, 6.4±0.06, respectively), cryptically coloured lizards, which produce visual displays that must be detected by territorial neighbours at considerable distances (up to 25 m away). To make matters worse, there is almost constant motion from windblown vegetation in the signal environment of both species and such environmental motion has been shown in laboratory experiments to reduce the ability of lizards to detect a moving stimulus (reviewed in Fleishman 1992). In principle, displaying lizards might increase the probability of being seen by exaggerating the components of their signals that contrast most strongly against background motion. We tested one possibility (Fleishman 1992; Peters & Evans 2003a), namely that lizards in the wild should produce high-speed displays when visual noise from moving vegetation is high.
2. Material and methods
(a) Data collection
We focused specifically on adult males as their territories are typically much larger than those of adult females (Stamps 1983). Hence, the neighbouring recipients of territorial male broadcast signals are typically located at farther distances than the recipients of female communication signals. We focused on head-bob patterns rather than the conspicuous dewlap colours of male anoles (e.g. Leal & Fleishman 2004) because dewlap colour is a static amplifier of a signal that is specifically dependent on movement (see movies in electronic supplementary material A). With sufficient luminance contrast, it is the movement of a displaying animal in the visual periphery that attracts the attention of distant receivers. Moreover, it is the characteristics of this movement relative to that occurring in the rest of the environment, which influences the likelihood of receivers detecting the display (Fleishman 1992; Peters & Evans 2003a).
A video library of display/background footage for male A. cristatellus and A. gundlachi was compiled from 15 to 22 April 2005 by recording lizards opportunistically in their natural habitat at various sites within walking distance of the El Verde Field Station inside the Caribbean National Forest. Both species are arboreal and males were generally found perched 1.5–2 m above the ground on large tree trunks. Lizards were located by quietly walking through the preferred habitat of each species: A. cristatellus were found on trees along the road edge of Route 186, at the forest edge of a disused picnic area and along a closed road leading into the Caribbean National Forest; A. gundlachi were found by walking transects leading into the forest behind the El Verde Field Station parallel to the Quebrada Sondora river. Areas where lizards had been recorded were not revisited to ensure that the same individuals were not sampled more than once.
When a male was spotted, a NTSC Panasonic mini digital video camcorder (PV-GS15 with ×20 optical zoom lens) was placed on a tripod approximately 4–5 m away at a height level with the focal animal. All trials were recorded on Panasonic DVM60ME digital tape. Lizards were initially recorded for 10 min. If the male did not display, the trial was aborted. In the majority of cases, however, lizards produced at least one broadcast display and the trial was continued for a further 20–25 min, or until the male moved out of view (average trial time: 28 min). A broadcast display was defined as any apparently spontaneous, non-directed head-bob sequence, which was almost always accompanied by the expansion of the dewlap. These displays, like broadcast signals in other territorial animals (e.g. birds; see §1), serve to advertise continued territory occupancy to both neighbours and more distant conspecifics. Interference from environmental noise is, therefore, a particular problem for this class of signal. Instances in which only the dewlap was moved were not included in analyses (speed estimates of dewlap movement were always lower than estimates of head-bobs and background movement). Lizards sometimes shifted perches during trials. If necessary, the camcorder was moved to ensure the profile of the lizard remained as close to side on to the camcorder as possible. At the end of all trials, a ping-pong ball of known size was video-recorded at each display site with the camcorder in its original position (which had been marked in cases where the camcorder had been moved during the trial). This allowed speed estimates to be calibrated to a unit of physical distance moved (see below).
(b) Motion analysis
Display sequences were imported from digital tapes off the camcorder through an IEEE 1394 ‘firewire’ cable connected to a PowerBook G4 apple computer and edited using iMovie HD 5.0.2. Clips were then exported as AVI movies using the compression type DV/DVCPRO-NTSC and compressor set at ‘best’. These were burnt to DVD and transferred to a Toshiba Tecra S2 PC for image analysis.
Speed was quantified using the MatLab-based program AIM v. 1.2 (‘Analysis of Image Motion’; Peters 2006). This approach has been described in detail elsewhere (Peters et al. 2002; Peters & Evans 2003b). Briefly, the program uses ‘biologically inspired’ motion detector algorithms developed for machine vision to quantify and compare complex movement-based displays (Zeil & Zanker 1997; Peters et al. 2002).
For each video clip, a polygon was defined around the area of the image traversed by the displaying lizard (determined manually by stepping through the clip every 15 frames). Output was subsequently partitioned by movement detected within and outside the polygon, allowing the calculation of separate estimates for display movement and moving vegetation occurring in the rest of the image. The velocity fields were reduced to a single estimate of motion strength for each frame; velocity magnitudes (speed) averaged after low-level motion estimates (below 0.2 mm s−1) were zeroed. Estimates for speed were then multiplied by a calibration factor in millimetres determined using an object of known size at the location of the displaying lizard (i.e. the ping-pong ball).
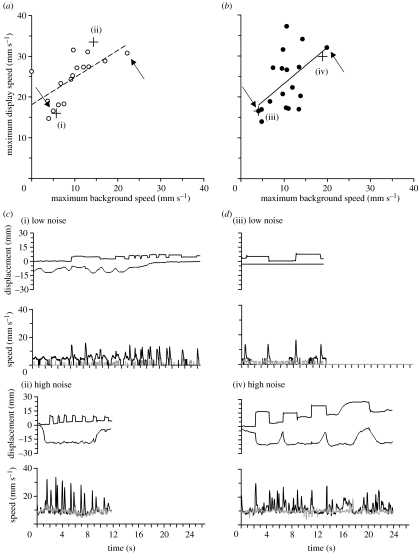
Output was presented as speed plots depicting changes in speed (defined as mm s−1) over time. From these plots, separate measures of maximum speed for the display and background movement were obtained (e.g. figure 1c,d). We used maximum speed rather than mean speed because inspection of speed plots revealed that some background movement was not entirely excluded from the region defined by the polygon around the displaying lizard. This meant that despite the lizard being stationary at some points during the display sequence (e.g. at the peak of a head-bob), speed estimates did not always drop to zero making a measure of average display speed potentially inflated for some clips. A measure of maximum speed removes this potential confound while still providing a biologically relevant parameter for signal detection—a burst of rapid movement in the visual periphery can be expected to be an effective trigger of the orienting response in an inattentive receiver. (Note: Regardless, the mean speed and maximum speed estimates for background movement are strongly correlated (n=34, r2=0.60) suggesting these same parameters for display movement would be as well.)
Figure 1.
The effect of background motion on the production of dynamic visual displays by males of two Puerto Rican anole species: (a) Anolis cristatellus and (b) A. gundlachi. Each species prefers a different habitat type—A. cristatellus occupy territories along road and forest edges, while A. gundlachi are found in deep shade forests—nevertheless both experience visual noise from windblown vegetation. We used computational motion analysis (Peters et al. 2002) to quantify lizard head-bobbing displays and background motion from digital video recorded in the field. During video analysis, the portion of the clip depicting the lizard was partitioned out from the rest of the image to enable separate estimates of display speed and background speed to be calculated. Each data point represents the mean values for a given individual. (c) Representative examples of two display sequences produced during (i) low visual noise and (ii) high visual noise for different individuals of A. cristatellus. (d) Similarly, representative examples for A. gundlachi during (iii) low visual noise and (iv) high visual noise. Top panels in each noise category show display–action–pattern graphs illustrating the movement of the head (upper line) and dewlap (lower line) over time. Bottom panels are corresponding speed plots for each sequence showing changes in speed over time for the display (black line) and in the background (grey line). The maximum speed (mm s−1) of displays and backgrounds (given in parenthesis) for each example were: (i) 15.97 (5.71); (ii) 33.53 (14.33); (iii) 16.53 (4.07); and (iv) 29.84 (18.89). The + symbol in scatter plots correspond to the speed estimates of each sequence shown in (c) and (d) while arrows indicate the mean data points that these values contributed to. The clips used for analyses depicted in d(iii, iv) are available as supplementary video clips (see movies in electronic supplementary material A).
Display–action–pattern graphs in figure 1 were created by recording frame-by-frame changes in the position of the eye and the leading edge of the dewlap, using the program NIH ImageJ v. 1.34.
(c) Statistical analysis
Measures of maximum display speed and maximum background speed were averaged within individuals to obtain a single variable estimate for each male. We used a Kolmogorov–Smirnov Test of Normality to confirm that data did not significantly deviate from a normal distribution (all tests, p>0.20). Maximum display speed was regressed on maximum background speed across individuals for each species separately using SPSS v. 11.5 (SPSS, Chicago, Illinois, USA).
To explore whether within-individual variability might have affected our findings, we developed a more complex regression model based on the following equation:
The first term b0, corresponds to the intercept, the following two terms corresponds to the underlying regression line and the last two terms corresponds to the within-lizard variability about that regression line. L refers to a given lizard and i to the ith observation for that lizard. The model was applied separately to each species using the PROC MIXED procedure in SAS v. 9.1.3 (SAS Institute, Cary, North Carolina, USA).
Our hypothesis predicts that display speed increases proportionally with background speed. Implicit in this prediction is that reductions in display speed in noisy motion environments will, in turn, make a display more difficult to detect. We, therefore, calculate one-tailed probability values for all correlations between display speed and background speed.
3. Results
We measured an average of 4 displays per individual for 16 adult male A. cristatellus (range: 1–9 displays per male) and 18 adult male A. gundlachi (range: 1–8). In both species, the average maximum display speed varied considerably between individuals (range: 14.7–31.5 and 14.0–37.2 mm s−1, respectively). We found that a significant proportion of this variance (25–45%) was explained by a strong positive correlation with the maximum speed of moving vegetation occurring in the background (linear regressions: A. cristatellus, n=16, r2=0.45, intercept=18.12 (95% CIs: 13.53–22.71), b=0.68 (0.24–1.09), p=0.0025, one-tailed; A. gundlachi, n=18, r2=0.25, intercept=14.37 (5.37–23.37), b=0.89 (0.07–1.70), p=0.0175, one-tailed; figure 1). That is, males experiencing high levels of visual background noise produced high-speed displays, while males experiencing calmer conditions produced more relaxed displays of lower speed.
It is possible that wind level varies by time of day and, in turn, corresponds with changes in ambient temperature affecting the speed at which displays are produced by these ectotherms. Although we did not record temperature during trials, we revisited the habitats of both species in April 2006 and collected temperature data as part of a different study. A quadratic regression showed that temperature and time are correlated (A. cristatellus habitat: n=19, r2=0.30, p=0.061; A. gundlachi habitat: n=21, r2=0.43, p=0.006). Nevertheless, in neither species did we find time of day related to the maximum speed of displays (quadratic regression: A. cristatellus, n=16 r2=0.12, p=0.426; A. gundlachi, n=18, r2=0.21, p=0.170) or the maximum speed of background motion (A. cristatellus: n=16 r2=0.10, p=0.514; A. gundlachi: n=18, r2=0.05, p=0.692). It is, therefore, unlikely that background noise and display speed are correlated owing to an underlying effect of time of day or temperature on both of these variables (see also below).
For both species, a regression model incorporating within-individual variability confirmed a positive relationship across individuals between the maximum speed of displays and the maximum speed of background motion (table 1). This model also indicated a positive relationship between these two variables across different displays produced by the same individual (table 1). These findings suggest that males adjusted the speed of their displays based on the level of background motion at the time of the display. It also supports the conclusion that time of day and temperature do not account for changes in display speed (e.g. temperature is unlikely to change appreciably during the 25–35 min of each trial).
Table 1.
Linear mixed models of maximum display speed on maximum background speed that incorporate within-individual variability. (Model estimates are calculated using restricted maximum likelihood.)
| effect | b | d.f. | t | p, one-tailed |
|---|---|---|---|---|
| Anolis cristatellus (n=16) | ||||
| intercept | 17.05 | 18.9 | 6.56 | <0.0001 |
| across individuals | 0.74 | 21.9 | 3.04 | 0.003 |
| within individuals | 0.32 | 58.8 | 2.22 | 0.015 |
| A. gundlachi (n=18) | ||||
| intercept | 14.65 | 16.7 | 3.22 | 0.003 |
| across individuals | 0.84 | 15.0 | 2.10 | 0.026 |
| within individuals | 0.73 | 2.29 | 4.48 | 0.018 |
4. Discussion
We show that adult male Anolis lizards in two different species produce territorial displays with motion characteristics that vary as a function of prevailing levels of visual background noise from windblown vegetation. At a proximal level, a relationship between display speed and background motion is consistent with two hypotheses. One possibility is that adult male display speed is relatively fixed by the time that a male is mature, e.g. as a result of the effects of genes, maternal effects or juvenile experience on display characteristics. In that case, a positive relationship between male display speed and habitat noise might occur because males select territories and habitats in which their displays are most likely to be effective. The possible effects of juvenile experience on adult display characteristics are consistent with observations that Anolis lizards of both sexes begin producing displays soon after hatching (e.g. Stamps 1978; Lovern & Jenssen 2003), and that anoles use these displays during territorial and aggressive interactions throughout their lives (Stamps & Barlow 1973; Jenssen 1977). Alternatively, displays may be plastic in adults, such that males are able to modify their display speeds over the short term, in conformance with current habitat conditions.
In the present study, we found indications that individual males changed display speed on a display-by-display basis in response to fluctuations in background speed. These results tend to support the hypothesis that display speed in adult males is plastic and that males have some ability to adjust their display speeds based on short-term changes in environmental noise conditions. Since our study was not explicitly designed to investigate changes in display speed within individuals, the results of this analysis, while statistically significant, should be taken as advisory rather than confirmatory. However, they do suggest that plasticity in display speed is clearly worth examining in future studies.
Territorial male broadcast signals must reach multiple adult male and female receivers located at different directions and distances from the male's perch. Males producing such signals do not have the option of shifting location for each display to minimize interference from background motion or of moving closer to a particular receiver to improve signal reception. Hence, even though the visual impact of rapidly moving vegetation varies as a function of the distance from the signaller to the vegetation and of the distance of the signaller to its recipients, it is impractical for males to adjust their location to avoid visual noise from background vegetation. Instead, a better strategy is to change the properties of the broadcast display to enhance its conspicuousness to any receivers that might be active in the male's neighbourhood. Our findings show that males of A. cristatellus and A. gundlachi do just that. Those experiencing high levels of visual noise from windblown vegetation typically produce broadcast displays of high speed. By increasing the speed of broadcast displays relative to the speed of habitat motion, lizards are more likely to enhance the transmission efficiency of broadcast displays.
Our findings provide the first example of a correlated relationship between background noise and the form of a dynamic visual display. In the same way that calling birds and other animals must compete with sound from windblown vegetation to be heard, visually displaying lizards must compete with visual noise from moving vegetation to be seen. As with animals communicating vocally, we show that lizards produce signal properties that are dependent on prevailing levels of habitat noise.
Acknowledgments
We thank Leo Fleishman, Jan Hemmi, Jonathan Losos, Jochen Zeil, members of the Patricelli laboratory and two anonymous reviewers for constructive comments on a previous version of the manuscript, Leo Fleishman and Jonathan Losos who also provided advice on data collection in the field and Neil Willits for statistical advice and SAS programming. We are also indebted to Alonzo Ramirez and the University of Puerto Rico for providing access to facilities and accommodation at the El Verde Field Station. This research was supported by grants from the National Geographic Society and US National Science Foundation (IOB-0517041) to T.J.O. and J.A.S., the Australian Research Council (DP-0557018) to R.A.P. and a graduate fellowship from the US National Science Foundation to B.C. The research described here was covered by the Animal Use and Care Protocol 05-11652 approved on 24 March 2005 by the Institutional Animal Care and Use Committee of the University of California, Davis.
Supplementary Material
A video clip of a male A. gundlachi experiencing low background noise and producing a relaxed display sequence. The data quantified from this clip is illustrated in iii of Fig. 1d
A video clip of a male A. gundlachi experiencing high background motion and producing a high-speed display sequence. The data quantified from this clip is illustrated in iv of Fig. 1d
References
- Aubin T, Jouventin P. Localization of an acoustic signal in a noisy environment: the display call of the king penguin Aptenodytes patagonicus. J. Exp. Biol. 2002;205:3793–3798. doi: 10.1242/jeb.205.24.3793. [DOI] [PubMed] [Google Scholar]
- Brumm H, Slabbekoorn H. Acoustic communication in noise. Adv. Study Behav. 2005;35:151–209. doi:10.1016/S0065-3454(05)35004-2 [Google Scholar]
- Brumm H, Voss K, Kollmer I, Todt D. Acoustic communication in noise: regulation of call characteristics in a New World monkey. J. Exp. Biol. 2004;207:443–448. doi: 10.1242/jeb.00768. doi:10.1242/jeb.00768 [DOI] [PubMed] [Google Scholar]
- Feng A.S, Narins P.M, Xu C.-H, Lin W.-Y, Yu Z.-L, Qiu Q, Xu Z.-M, Shen J.-X. Ultrasonic communication in frogs. Nature. 2006;440:333–336. doi: 10.1038/nature04416. doi:10.1038/nature04416 [DOI] [PubMed] [Google Scholar]
- Fleishman L.J. The influence of the sensory system and the environment on motion patterns in the visual displays of anoline lizards and other vertebrates. Am. Nat. 1992;139:S36–S61. doi:10.1086/285304 [Google Scholar]
- Fox S.F, McCoy J.K, Baird T.A. John Hopkins University Press; Baltimore, MD: 2003. Lizard social behavior. [Google Scholar]
- Jenssen T.A. Evolution of anoline lizard display behavior. Am. Zool. 1977;17:203–215. [Google Scholar]
- Lane H, Tranel B. The Lombard sign and the role of hearing in speech. J. Speech Hear. Res. 1971;14:677–709. [Google Scholar]
- Langemann U, Gauger B, Klump G.M. Auditory sensitivity in the great tit: perception of signals in the presence and absence of noise. Anim. Behav. 1998;56:763–769. doi: 10.1006/anbe.1998.0879. doi:10.1006/anbe.1998.0879 [DOI] [PubMed] [Google Scholar]
- Leal M, Fleishman L.J. Differences in visual signal design and detectability between allopatric populations of Anolis lizards. Am. Nat. 2004;163:26–39. doi: 10.1086/379794. doi:10.1086/379794 [DOI] [PubMed] [Google Scholar]
- Lengagne T, Slater P.J.B. The effects of rain on acoustic communication: tawny owls have good reason for calling less in wet weather. Proc. R. Soc. B. 2002;269:2121–2125. doi: 10.1098/rspb.2002.2115. doi:10.1098/rspb.2002.2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengagne T, Aubin T, Lauga J, Jouventin P. How do king penguins (Atenodytes patagonicus) apply the mathematical theory of information to communicate in windy conditions? Proc. R. Soc. B. 1999;266:1623–1628. doi:10.1098/rspb.1999.0824 [Google Scholar]
- Leonard M.L, Horn A.G. Ambient noise and the design of begging signals. Proc. R. Soc. B. 2005;272:651–656. doi: 10.1098/rspb.2004.3021. doi:10.1098/rspb.2004.3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr B, Wright T.F, Dooling R.J. Detection and discrimination of natural calls in masking noise by birds: estimating the active space of a signal. Anim. Behav. 2003;65:763–777. doi:10.1006/anbe.2003.2093 [Google Scholar]
- Lovern M.B, Jenssen T.A. Form emergence and fixation of head bobbing displays in the green anole lizard (Anolis carolinensis): a reptilian model of signal ontogeny. J. Comp. Psychol. 2003;117:133–141. doi: 10.1037/0735-7036.117.2.133. doi:10.1037/0735-7036.117.2.133 [DOI] [PubMed] [Google Scholar]
- Naguib M, Wiley R.H. Estimating the distance to a source of sound: mechanisms and adaptations for long-range communication. Anim. Behav. 2001;62:825–837. doi:10.1006/anbe.2001.1860 [Google Scholar]
- Patricelli G.L, Blickley J. Avian communication in urban noise: causes and consequences of vocal adjustment. Auk. 2006;123:639–649. doi:10.1642/0004-8038(2006)123[639:ACIUNC]2.0.CO;2 [Google Scholar]
- Peters, R. A. 2006 Analysis of image motion. Available free from author.
- Peters R.A, Evans C.S. Introductory tail-flick of the jacky dragon visual display: signal efficacy depends upon duration. J. Exp. Biol. 2003a;206:4293–4307. doi: 10.1242/jeb.00664. doi:10.1242/jeb.00664 [DOI] [PubMed] [Google Scholar]
- Peters R.A, Evans C.S. Design of the jacky dragon visual display: signal and noise characteristics in a complex moving environment. J. Comp. Physiol. A. 2003b;189:447–459. doi: 10.1007/s00359-003-0423-1. doi:10.1007/s00359-003-0423-1 [DOI] [PubMed] [Google Scholar]
- Peters R.A, Clifford C.W.G, Evans C.S. Measuring the structure of dynamic visual signals. Anim. Behav. 2002;64:131–146. doi:10.1006/anbe.2002.3015 [Google Scholar]
- Pytte C, Rusch K.M, Ficken M.S. Regulation of vocal amplitude by the blue-throated hummingbird, Lampornis clemenciae. Anim. Behav. 2003;66:703–710. doi:10.1006/anbe.2003.2257 [Google Scholar]
- Rabin L.A, McCowan B, Hooper S.L, Owings D.H. Anthropogenic noise and its effect on animal communication: an interface between comparative psychology and conservation biology. Int. J. Psychol. 2003;16:172–192. [Google Scholar]
- Ryan M.J, Brenowitz E.A. The role of body size, phylogeny, and ambient noise in the evolution of bird song. Am. Nat. 1985;126:87–100. doi:10.1086/284398 [Google Scholar]
- Ryan M.J, Cocroft R.B, Wilczynski W. The role of environmental selection in intraspecific divergence of mate recognition signals in the cricket frog, Acris crepitans. Evolution. 1990;44:1869–1872. doi: 10.1111/j.1558-5646.1990.tb05256.x. doi:10.2307/2409514 [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Peet M. Birds sing at a higher pitch in urban noise. Nature. 2003;424:267. doi: 10.1038/424267a. doi:10.1038/424267a [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Smith T.B. Habitat-dependent song divergence in the little greenbul: an analysis of environmental selection pressures on acoustic signals. Evolution. 2002;56:1849–1858. doi: 10.1111/j.0014-3820.2002.tb00199.x. doi:10.1554/0014-3820(2002)056[1849:HDSDIT]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stamps J.A. A field study of the ontogeny of social behaviour in the lizard Anolis aeneus. Behaviour. 1978;66:1–31. [Google Scholar]
- Stamps J.A. Sexual selection, sexual dimorphism, and territoriality. In: Huey R.B, Pianka E.R, Schoener T.W, editors. Lizard ecology: studies of a model organism. Harvard University Press; Cambridge, MA: 1983. pp. 169–204. [Google Scholar]
- Stamps J.A, Barlow G.W. Variation and stereotypy in the displays of Anolis aeneus (Sauria: Iguanidae) Behaviour. 1973;47:67–94. [Google Scholar]
- Wiley R.H, Richards D.G. Adaptations for acoustic communication in birds: sound transmission and signal detection. In: Kroodsma D.E, Miller E.H, Ouellet H, editors. Acoustic communication in birds. Academic Press; New York, NY: 1982. pp. 131–181. [Google Scholar]
- Witte K, Farris H.E, Ryan M.J, Wilczynski W. How cricket frog females deal with a noisy world: habitat-related differences in auditory tuning. Behav. Ecol. 2005;16:571–579. doi:10.1093/beheco/ari032 [Google Scholar]
- Zeil J, Zanker J.M. A glimpse into crab world. Vision Res. 1997;37:3417–3426. doi: 10.1016/s0042-6989(97)00106-5. doi:10.1016/S0042-6989(97)00106-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video clip of a male A. gundlachi experiencing low background noise and producing a relaxed display sequence. The data quantified from this clip is illustrated in iii of Fig. 1d
A video clip of a male A. gundlachi experiencing high background motion and producing a high-speed display sequence. The data quantified from this clip is illustrated in iv of Fig. 1d