Abstract
Many collapsed fish populations have failed to recover after a decade or more with little fishing. This may reflect evolutionary change in response to the highly selective mortality imposed by fisheries. Recent experimental work has demonstrated a rapid genetic change in growth rate in response to size-selective harvesting of laboratory fish populations. Here, we use a 30-year time-series of back-calculated lengths-at-age to test for a genetic response to size-selective mortality in the wild in a heavily exploited population of Atlantic cod (Gadus morhua). Controlling for the effects of density- and temperature-dependent growth, the change in mean length of 4-year-old cod between offspring and their parental cohorts was positively correlated with the estimated selection differential experienced by the parental cohorts between this age and spawning. This result supports the hypothesis that there have been genetic changes in growth in this population in response to size-selective fishing. Such changes may account for the continued small size-at-age in this population despite good conditions for growth and little fishing for over a decade. This study highlights the need for management regimes that take into account the evolutionary consequences of fishing.
Keywords: exploited populations, evolution, fisheries, heritability, quantitative genetics, size selection
1. Introduction
Fisheries have been described as large-scale uncontrolled experiments in evolution (Rijnsdorp 1993; Stokes & Law 2000). The mortality imposed by many fisheries is high, often two or three times the level of natural mortality, and strongly selective, typically targeting larger individuals. Yet, the possibility of genetic change in exploited fish populations in response to the strong selection generated by fishing is ignored in fisheries management. This may reflect the traditional view that evolution is a slow process not discernable over the short ecological time-scales relevant to resource managers (Reznick & Ghalambor 2005). However, recent work has demonstrated that evolution can be rapid, with significant change occurring within years or decades rather than centuries (Reznick et al. 1997; Thompson 1998; Koskinen et al. 2002; Stockwell et al. 2003).
Many collapsed fish populations have failed to recover even when fishing mortality has been reduced after their collapse (Hutchings 2000; Hutchings & Reynolds 2004). This may reflect fisheries-induced genetic changes in traits related to population productivity (Hutchings & Reynolds 2004; Hutchings 2005; Walsh et al. 2006). Decline in age and size at maturity, the predicted evolutionary response to the increased mortality imposed by fisheries, has been demonstrated in a growing number of cases and appears to reflect genetic change (e.g. Grift et al. 2003; Olsen et al. 2004; Barot et al. 2004; but see Morita & Fukuwaka 2006). Evolutionary decline in growth rate has also been predicted as a response to the selective removal of fast-growing individuals by fisheries (Law 2000; Stokes & Law 2000; Conover & Munch 2002). Rapid genetic changes in growth rate and correlated life-history traits in response to size-selective harvesting have been demonstrated in laboratory populations of the Atlantic silverside, Menidia menidia (Conover & Munch 2002; Walsh et al. 2006). Here, we present results consistent with a genetic response to size-selective mortality in the wild in a heavily exploited fish population: Atlantic cod (Gadus morhua) in the southern Gulf of St Lawrence.
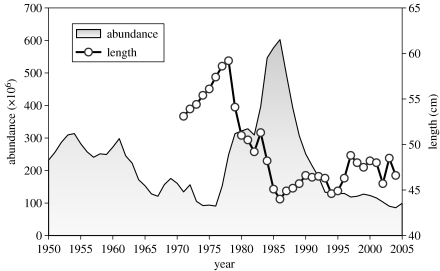
Cod have been fished in the Gulf of St Lawrence since the sixteenth century. Fishery removals from the southern Gulf population increased sharply in the 1950s, and the population declined throughout the 1960s and early 1970s (figure 1). The stock recovered quickly in the late 1970s and early 1980s despite continued fishing. However, the stock collapsed again in the late 1980s and has not yet recovered, even though there has been little fishing since 1993. This difference in recovery rates between the 1970s and 1990s reflects a change in the productivity of this population. Productivity has the following three components in terms of the rate of change in spawning stock biomass: the rate of recruitment of juveniles to the spawning stock; body growth rate; and the natural mortality rate of adults. Changes in all the three components contributed to the low productivity of this population in the 1990s (Shelton et al. 2006). Here, we focus on the changes in body growth rate. Length-at-age in this population decreased sharply in the late 1970s and early 1980s (figure 1). This reflected a density-dependent decline in growth rate combined with a change in the direction of size-selective fishing mortality (Sinclair et al. 2002b). The fishery selectively removed slow-growing individuals in the early to mid-1970s and fast-growing individuals from the early 1980s until the closure of directed fishing in 1993 (Hanson & Chouinard 1992; Sinclair et al. 2002a).
Figure 1.
Abundance of cod aged 4 years and older in the southern Gulf of St Lawrence and mean length at age 6 years. Estimates of abundance and mean length are taken from Chouinard et al. (2005). Abundance estimates were obtained by the calibration of an age-structured population model.
The changes in size selection observed in this population are thought to reflect the strongly dome-shaped relationship between fishing mortality of southern Gulf cod and their length, with the highest fishing mortality at intermediate lengths (Sinclair et al. 2002a). This creates potential fitness benefits associated with both fast and slow growth. Slow-growing fish have the advantage that they delay their vulnerability to the fishery. Fast-growing fish have the advantage that they grow more quickly through the window of lengths vulnerable to the fishery. The balance of selection will depend on the average growth rates in the population relative to the window of lengths vulnerable to the fishery. In the late 1970s and early 1980s, mesh size in the fishery increased (shifting the window of vulnerability to larger lengths) and growth rate decreased. These changes coincided with shifts in the balance of size selection of southern Gulf cod, from a period when the survival was lowest for slow-growing fish, through a period of disruptive selection when survival was lowest for fish with intermediate growth rates, to a period beginning in the early 1980s when the survival was lowest for fast-growing fish (Sinclair et al. 2002a).
Length-at-age of southern Gulf cod remained low throughout the 1990s and early 2000s, even though conditions for growth were good (low cod abundance, high prey abundance and warm ambient temperatures during the feeding season) and size-selective fishery removals were low. A possible explanation is that there has been a genetic response to the selective removal of fast-growing fish throughout the 1980s and early 1990s (Sinclair et al. 2002a). Here, we test this hypothesis using a quantitative genetics model applied to data on length-at-age back-calculated from otoliths.
2. Material and methods
Data are from stratified random bottom-trawl surveys conducted in the southern Gulf of St Lawrence each September since 1971. Otoliths were extracted from length-stratified subsamples of the cod captured in each tow. Otoliths were sectioned and ages were determined by counting the number of hyaline zones on the otolith cross-section. The distance from the nucleus of the otolith to the outside edge of each hyaline zone was measured along the longest axis of the cross-section. This edge, termed the annulus, corresponds with the end of the winter period of slow growth, which occurs in April in this population. Lengths-at-age were back-calculated using the biological intercept method (Campana 1990),
| (2.1) |
where li is the estimated fish length at age i; lC is the fish length at capture; Oi is the otolith radius at annulus i; OC is the total otolith radius and l0 and O0 are the fish length and otolith radius at the biological intercept, respectively, defined as the point at which fish and otolith growth become proportional. Further details are given by Sinclair et al. (2002a). Back-calculations were available for the period 1971–2001. Analyses presented here are restricted to cohorts produced in 1977–1997 and the parents that produced these cohorts (a cohort refers to the group of fish born in a particular year).
The trait examined in this study was the back-calculated length at the fourth annulus, corresponding to an age of 4 years. This age was chosen as a compromise between an age well recruited to the survey and one poorly recruited to the fishery. At this age, cohorts are fully recruited to the research survey so that size composition is representatively sampled (Sinclair et al. 2002b). However, at an age of 4 years, cohorts have experienced little fishing mortality and thus little fishery-induced phenotypic selection, at least for cohorts produced since 1977 (Chouinard et al. 2005).
Let Li,j be the mean back-calculated length at the fourth annulus in the cohort produced in year j (referred to here as cohort j) for fish observed at age i in the September survey. We calculated Li,j weighting observations based on the stratified sampling scheme, as described by Sinclair et al. (2002a). The dependent variable in this analysis was ΔL4,j, the L4,j for offspring cohort j minus that for the spawning stock that produced it,
| (2.2) |
where amin and amax are the minimum and maximum ages used for the spawning stock, respectively, and pi,j−i is the proportion of the spawning stock biomass in year j comprising parental cohort j−i. Back-calculations were available for fish up to an age of 11 years, so the portion of the spawning stock older than this age was excluded from the analysis. The excluded ages averaged less than 4% of the spawner biomass in the 1977–1997 period. The small portion (1%) of the spawning stock biomass comprising 3-year-olds was also excluded from this calculation (because the trait examined here is not expressed at this age). No estimate was available for L4,1966, so calculations for 1977 excluded 11-year-old spawners (the 1966 cohort, comprising 4% of the spawner biomass in that year). The relative contribution of each age-class to spawner biomass was based on the September survey data. Spawning in southern Gulf cod occurs mainly between late May and mid-July; prior to the closure of directed fishing in 1993, the proportion of the annual fishing mortality that occurred between spawning and September was low (Sinclair et al. 1994).
We used the following model to test for a genetic change in L4,j in response to size selection:
| (2.3) |
where ΔL4,j is the difference between offspring cohort j and its parental cohorts in mean back-calculated length at age 4 years in fish observed as 4-year-olds; ΔGj represents the contribution of genetic change to this difference; and ΔEj is the contribution of phenotypic plasticity (i.e. environmental effects). ΔGj is the effect of genetic change in the parental cohorts between age 4 years (when their L4,j−i is measured) and their production of offspring cohort j. This genetic change is the evolutionary response to the size selection experienced by parents between age 4 years and spawning. Following Falconer (1981), the response to selection is given by
| (2.4) |
where h2 is the heritability of length at age 4 years and is the average selection differential for this length in the spawning stock that produced offspring cohort j. Si,j−i, the selection differential for parental cohort j−i (aged i in the spawning stock in year j), is the change in the mean length at the fourth annulus between the fish observed at age 4 years and those observed at age i,
| (2.5) |
The average selection differential for the parents of offspring cohort j is given by
| (2.6) |
Note that S4,j−4 is by definition 0. S3,j−3 was also set to 0.
ΔE is the effect of differences between offspring and their parents in the environmental conditions experienced up to age 4 years. The following two factors were considered in the ΔE term: the differences in density experienced by parents and offspring (Δd) and the differences in temperature conditions experienced up to age 4 years (Δt).
Δd was included in the analysis to account for the effects of density-dependent growth. Density-dependent growth reflects intraspecific competition, which is expected to be the strongest between individuals of similar sizes (and thus similar diets; see Hanson & Chouinard (2002) for information on length-specific diets of southern Gulf cod). Because length overlaps broadly between adjacent age classes in this population, the density index constructed for cohort j was based on the abundance of that cohort and the abundances of the preceding and following cohorts:
| (2.7) |
where NJ is the year-class strength for cohort J, defined as the abundance of 3-year-old cod in year J+3. Estimates of abundance were from the sequential population analysis reported by Chouinard et al. (2005). The difference in density between offspring cohort j and its parent cohorts, Δdj, was calculated as
| (2.8) |
An index of the ambient temperature of cod during the feeding season was constructed to test for the effects of temperature-dependent growth. This index was based on tow-by-tow bottom temperatures during the September surveys weighted by cod catch. These surveys provide synoptic estimates of the distribution of cod with respect to temperature during the feeding season, a time of year when southern Gulf cod are non-migratory (Clay 1991). For cod of age i in year y, ambient temperature was estimated as the stratified mean cod-weighted bottom temperature
| (2.9) |
where vs,y is the weighting factor associated with tow s in year y; Ys,i,y is the number of cod of age i caught in tow s in year y; is the stratified mean catch of age i cod per tow in year y (calculated using the weights vs,y); τs,y is the bottom temperature during tow s in year y; and n is the number of tows in year y. The weighting factor vs,y was the proportion of the survey area covered by the stratum fished by tow s divided by the number of sites fished in that stratum in year y. For cohort j, the temperature index was the average ambient temperature for ages 1–3 years,
| (2.10) |
The difference in ambient temperature between offspring cohort j and its parental cohorts, Δtj, was calculated as
| (2.11) |
Data on ambient temperature of cod are available since 1971. Thus, estimates of at least one of the ambient temperatures for ages 1–3 years were not available for the oldest ages in the spawning stocks in 1977–1980 (e.g. ambient temperature at ages 1–3 years for the 1966 cohort aged 11 years in 1977, ambient temperature at age 1 year for the 1969 cohort aged 11 years in 1980). The average ambient temperature at age was used in these cases (3% of the values needed to calculate the time-series of Δt).
A general index of environmental conditions was also constructed. This index was based on the average bottom temperature in the September surveys without weighting by cod catch. However, ΔL4,j was unrelated to the differences in this index between offspring and their parental cohorts, and results of tests using this index are not presented here.
Regression analyses presented in the report were conducted using the function lm in S-Plus 2000 (MathSoft, Inc. 1999). Δd and Δt were standardized by subtracting their mean and dividing by their standard deviation prior to their use as independent variables in the regression analyses. Hierarchical models including S, Δd and/or Δt were compared using the small sample version of Akaike's information criterion (AICc, Burnham & Anderson 2002). The model with the lowest AICc is estimated to be the most parsimonious of the models considered. Δi, the AICc of model i minus the minimum AICc of the considered models, is a measure of the empirical support for model i. Values of 0–2 for Δi indicate considerable support, whereas values of 4–7 indicate considerably less support (Burnham & Anderson 2002). The likelihood that a model is the best model, given the data and the set of models considered, is given by its Akaike weight, w (Burnham & Anderson 2002, p. 75).
3. Results
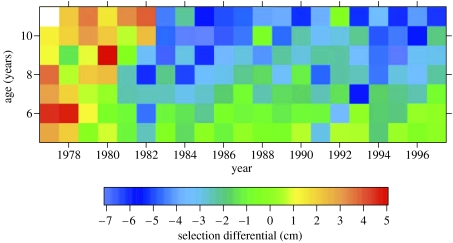
Selection differentials for length at age 4 years differed between the parents of cohorts produced in the late 1970s and those produced between the early 1980s and the mid-1990s (figures 2 and 3a, table S1 in the electronic supplementary material). Selection differentials were positive, indicating greater survival for fast-growing fish, for the parents of cohorts produced in the earlier period and negative, indicating greater survival for slow-growing fish, for the parents of cohorts produced in the later period.
Figure 2.
Selection differentials for length at age 4 years for the spawning stocks producing the 1977–1997 cohorts.
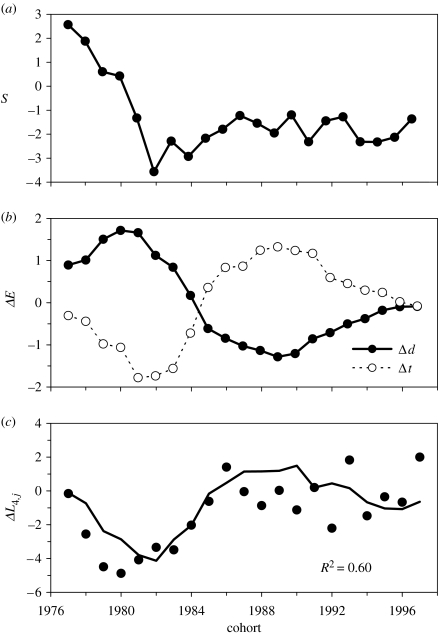
Figure 3.
Interannual variation in ΔL4,j and potential explanatory variables. (a) S, the average selection differential for length at age 4 years in the spawning stocks producing the 1977–1997 cohorts. (b) Differences in density (Δd) and temperature conditions experienced up to age 4 years (Δt) between offspring and parental cohorts, standardized to a mean of 0 and a standard deviation of 1. (c) Observed variation in ΔL4,j (circles) and that predicted by model 2 in table 1 (line). R2 is the proportion of the variation in ΔL4,j explained by model 2.
The two environmental factors were strongly confounded (figure 3b). Density was greater for offspring than for their parents for cohorts produced in the late 1970s and early 1980s and the reverse for those produced from the mid-1980s to the early 1990s. Differences in ambient temperature between offspring and their parents showed the opposite pattern, with cohorts produced in the late 1970s and early 1980s experiencing lower temperatures at ages 1–3 years than their parents and those produced from the mid-1980s to the early 1990s experiencing higher temperatures than their parents. The close correspondence between these two trends reflects density-dependent temperature associations in this population, with a greater proportion of the population occupying relatively cold waters when abundance is high (Swain & Kramer 1995). Density and temperature are generally expected to have opposite effects on growth, with growth depressed by high density or low temperature. Thus, given their opposing temporal trends, the effect of each environmental factor is expected to reinforce the effect of the other on the difference in growth between parents and offspring.
Models that included terms for both a genetic response to selection (S) and an effect of differences in density between offspring and their parents (Δd) were much more probable given the data than all models that did not include both these terms (table 1). The model most strongly supported by the data included both these terms and a term for the effect of temperature differences between offspring and their parents (model 1 in table 1). However, based on AICc, the model that included only terms for S and Δd (model 2) was almost as probable as model 1 (table 1). Both the genetic response to selection and the effect of density were highly significant and in the expected directions in models 1 and 2 (table 2).
Table 1.
Support for models predicting ΔL4,j from S, Δd and/or Δt. (ΔL4,j is the change between offspring cohort j and its parental cohorts in mean back-calculated length at age 4 years in fish observed as 4-year-olds; S is the selection differential experienced by the parental cohorts between age 4 years and spawning; Δd and Δt are the differences in density and temperature conditions between offspring and parental cohorts; Δi is the AICc of model i minus the minimum AICc of the considered models; and wi is the Akaike weight for model i.)
| model | R2 | Δi | wi |
|---|---|---|---|
| 1: ΔL4,j=β1S+β2Δd+β3Δt | 0.66 | 0.0 | 0.5570 |
| 2: ΔL4,j=β1S+β2Δd | 0.60 | 0.7 | 0.3945 |
| 3: ΔL4,j=β1S+β3Δt | 0.47 | 6.4 | 0.0222 |
| 4: ΔL4,j=β2Δd+β3Δt | 0.37 | 10.1 | 0.0036 |
| 5: ΔL4,j=β2Δd | 0.37 | 7.3 | 0.0142 |
| 6: ΔL4,j=β3Δt | 0.34 | 8.4 | 0.0083 |
| 7: ΔL4,j=β1S | 0.07 | 15.6 | 0.0002 |
Table 2.
Effects of a genetic response to selection (S) and environmental differences between offspring and parents (Δd, density differences; Δt, temperature differences) on ΔL4,j, the change between offspring cohort j and its parental cohorts in mean back-calculated length at age 4 years in fish observed as 4-year-olds, for selected models. (β is the parameter estimate for an effect and s.e. its standard error. Significance levels (p) are from two-sided tests using type III sums of squares.)
| model | effect | β | s.e. | t | p |
|---|---|---|---|---|---|
| 1 | S | 0.758 | 0.191 | 3.96 | 0.0009 |
| Δd | −4.176 | 1.299 | −3.22 | 0.0048 | |
| Δt | −2.360 | 1.253 | −1.88 | 0.076 | |
| 2 | S | 0.587 | 0.179 | 3.27 | 0.0040 |
| Δd | −1.817 | 0.364 | −4.99 | 0.0001 | |
| 3 | S | 0.434 | 0.198 | 2.19 | 0.042 |
| Δt | 1.528 | 0.403 | 3.80 | 0.0012 |
Because the two environmental factors were strongly confounded, it was difficult to distinguish between the effects of each. The effect of temperature was highly significant and in the commonly expected positive direction in model 3, which included terms for S and Δt (table 2). However, there was considerably less support for this model than for models 1 or 2. In model 1, which also included a term for Δd, the effect of temperature was neither significant (at the 0.05 level) nor in the expected direction. Owing to this and given the similar levels of support for models 1 and 2 based on AICc, our preferred model is model 2. This model accounted for 60% of the variation in the length difference at age 4 between offspring and parental cohorts based on a genetic response to the selection experienced by parents and density differences between parents and offspring at ages up to 4 years (figure 3c).
4. Discussion
We have related changes in length-at-age between parental cohorts and their offspring to an index expected to be positively correlated with genetic changes in growth (i.e. the selection differential), as well as indices for the non-genetic factors previously demonstrated to affect growth in this population (i.e. density and temperature). Controlling for the effects of density- and temperature-dependent growth, the mean length of 4-year-old cod in offspring cohorts minus that in their parental cohorts was positively correlated with the estimated selection differential experienced by parental cohorts between this age and spawning. This result supports the hypothesis that there have been genetic changes in growth in this population in response to size-selective fishing. However, as in any observational study of natural populations, we cannot rule out the possibility that the effects attributed to factors included in our models instead reflect the effects of confounded but untested factors. Other factors that may affect the growth of southern Gulf cod include variation in the duration of the feeding season (Comeau et al. 2002) and variation in prey abundance or quality. Indices that would permit the inclusion of these factors in our models do not exist at this time.
The effects of environmental factors on the growth differences between offspring and parental cohorts observed in this study are consistent with other analyses of the factors affecting growth in this population. Similar to the results of this study, Sinclair et al. (2002b) observed a strong negative effect of density and a weak positive effect of temperature on lengths at ages 5–11 years in this population. However, in their study, the strongest effect on length at these older ages was the phenotypic response to size-selective mortality. Sinclair et al. (2002b) did not test for effects on length at age 4 years, but did include L4,j as an explanatory variable in their models in order to account for variability in growth at early ages. Phenotypic responses to size-selective fishing are not expected to be an important source of variation in length at age 4 years in this population, because cohorts have experienced little fishing mortality at this age. However, the results presented here indicate that genetic changes in growth in response to the selection differentials acting on parental cohorts appear to be a component of changes in length at early ages in southern Gulf cod.
Our models are simplifications in many respects. For example, genotype–environment interactions are not incorporated in our models, nor are any nonlinearities in environmental effects. Like most other models used to examine the factors affecting cod growth (cf. Swain et al. 2003), our models cannot account for the interactive effects of temperature and food ration, in which an increase in temperature will accelerate growth if food is not limiting but may reduce growth if it is (e.g. Brett et al. 1969). Our selection differentials and environmental indices also do not take into account any variation in reproductive success (i.e. the number of progeny surviving to age 4 years) within age classes in the spawning stock. For example, if reproductive success within age classes is positively correlated with length, then realized selection differentials will be less negative or more positive than our estimated differentials. However, in trials in which we calculated selection differentials assuming that reproductive success within age classes was proportional to length, conclusions were similar to those reported here, with models 1 and 2 identified as the best models based on AICc.
The selection differentials used in this study incorporate any size selection due to natural mortality, as well as the effects of size-selective fishing. However, fisheries-induced size selection is clearly a major component of these selection differentials. The selection differentials calculated from back-calculated length-at-age were positive for the spawning stocks in the late 1970s and negative for those since the early 1980s. Based on comparisons between the length distributions at age in the fishery catch and in the research survey population, Hanson & Chouinard (1992) demonstrated the same change in size selection by the fishery.
The slope of the selection-differential term in our models provides an estimate of the heritability of length at age 4 years for the southern Gulf cod population. The estimate based on our preferred model is 0.59 (s.e. 0.18). Mousseau & Roff (1987) reported a median value of about 0.35 for the heritability of morphological traits like body size in ectotherms. In a review of the literature on selective breeding for aquaculture, Law (2000) reported a mean heritability for length-at-age of 0.30 (s.d. 0.21). Thus, though within the range of values reported for this type of trait (Mousseau & Roff 1987), our estimate of heritability of size-at-age in southern Gulf cod is higher than the average value reported for this trait. High heritability may reflect high mutational variability, low residual variances (e.g. environmental variance) and/or protection from selection by opposing or fluctuating selection pressures (Merilä & Sheldon 1999). The recent history of selection for size-at-age in this population has included a change in the direction of selection as well as an episode of disruptive selection (Sinclair et al. 2002a), patterns of selection that may contribute to the maintenance of genetic variation in this population (cf. Falconer 1981; Ellner & Hairston 1994). Unlike most of the estimates reported by Mousseau & Roff (1987), our estimate of heritability is based on the response to selection and may be biased for a variety of reasons (Falconer 1981), in particular by any environmental effects on growth that are confounded with S but not accounted for in our models.
Our results support the hypothesis that there has been an evolutionary response in the southern Gulf cod population to size-selective fishing mortality. The continued small size-at-age in this population over a decade after the cessation of heavy fishing despite good conditions for growth, may thus reflect a genetic decline in growth in response to the selective removal of fast-growing fish in the 1980s and early 1990s. Such a genetic change may not be readily reversible by limiting fishing mortality (Law 2000; Stokes & Law 2000; Conover et al. 2005; Walsh et al. 2006). Natural agents of selection are not likely to produce selection differentials in the reverse direction that are as strong as those that were produced by fishing. Fishing mortality experienced by this population in the 1980s and early 1990s considerably exceeded the levels expected for natural mortality (Chouinard et al. 2005). Furthermore, because natural selection for growth is moderated by evolutionary trade-offs (Billerbeck et al. 2001; Lankford et al. 2001), it may not normally be as strong as the selection that can be imposed by fisheries.
Rapid evolutionary responses to size-selective harvesting have been demonstrated in laboratory experiments involving severe selection and a model species with a short generation time (Conover & Munch 2002; Walsh et al. 2006). Results presented here suggest that evolutionary responses to fishing can also be rapid in long-lived species in the wild, where fishery-induced selection is less precise and may be opposed by evolutionary trade-offs. This adds further weight to the case for adopting an evolutionary perspective in fisheries management (e.g. Law & Grey 1989; Heino 1998; Ernande et al. 2004; Conover et al. 2005). Management regimes that take into account the evolutionary consequences of fishing are needed in order to develop sustainable fisheries.
Acknowledgments
We thank L. Currie and J. Murphy for many hours of careful work measuring and ageing otoliths and J. A. Hutchings, C. J. Foote, G. A. Chouinard, E. M. P. Chadwick and two anonymous reviewers for their helpful comments on earlier versions of this manuscript.
Supplementary Material
Estimated selection differentials (cm) for length at age 4 yr for the spawning stocks producing the 1977 ? 1997 cohorts of Atlantic cod (Gadus morhua) in the southern Gulf of St. Lawrence (presented in Figure 2)
References
- Barot S, Heino M, O'Brien L, Dieckmann U. Long-term trend in the maturation reaction norm of two cod stocks. Ecol. Appl. 2004;14:1257–1271. [Google Scholar]
- Billerbeck J.M, Lankford T.E, Jr, Conover D.O. Evolution of intrinsic growth and energy acquisition rates. I. Trade-offs with swimming performance in Menidia menidia. Evolution. 2001;55:1863–1872. doi: 10.1111/j.0014-3820.2001.tb00835.x. doi:10.1554/0014-3820(2001)055[1863:EOIGAE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brett J.R, Shelbourn J.E, Shoop C.T. Growth rate and body composition of fingerling sockeye salmon Oncorhynchus nerka, in relation to temperature and ration size. J. Fish. Res. Board Can. 1969;26:2363–2394. [Google Scholar]
- Burnham K.P, Anderson D.R. 2nd edn. Springer; New York, NY: 2002. Model selection and multimodel inference. [Google Scholar]
- Campana S.E. How reliable are growth back-calculations based on otoliths? Can. J. Fish. Aquat. Sci. 1990;47:2219–2227. [Google Scholar]
- Chouinard G.A, Currie L.G, Poirier G.A, Swain D.P, Benoît H.P, Hurlbut T, Daigle D, Savoie L. Assessment of the southern Gulf of St. Lawrence cod stock, February 2005. DFO Can. Sci. Adv. Sec. Res. Doc. 2005;2005/007:95. [Google Scholar]
- Clay, D. 1991. Seasonal distribution of demersal fish (Osteichthyes) and skates (Chondrichthyes) in the southeastern Gulf of St. Lawrence. In The Gulf of St. Lawrence: small ocean or big estuary? (ed. J.-C. Therriault), Can. Spec. Publ. Fish. Aquat. Sci 113, 241–259.
- Comeau L.A, Campana S.E, Chouinard G.A. Timing of Atlantic cod (Gadus morhua L.) seasonal migrations in the southern Gulf of St Lawrence: interannual variability and proximate control. ICES J. Mar. Sci. 2002;59:333–351. doi:10.1006/jmsc.2001.1153 [Google Scholar]
- Conover D.O, Munch S.B. Sustaining fisheries yields over evolutionary time scales. Science. 2002;297:94–96. doi: 10.1126/science.1074085. doi:10.1126/science.1074085 [DOI] [PubMed] [Google Scholar]
- Conover D.O, Arnott S.A, Walsh M.R, Munch S.B. Darwinian fishery science: lessons from the Atlantic silverside (Menidia menidia) Can. J. Fish. Aquat. Sci. 2005;62:730–737. doi:10.1139/f05-069 [Google Scholar]
- Ellner S, Hairston N.G., Jr Role of overlapping generations in maintaining genetic variation in a fluctuating environment. Am. Nat. 1994;143:403–417. doi:10.1086/285610 [Google Scholar]
- Ernande B, Dieckmann U, Heino M. Adaptive changes in harvested populations: plasticity and evolution of age and size at maturation. Proc. R. Soc. B. 2004;271:415–423. doi: 10.1098/rspb.2003.2519. doi:10.1098/rspb.2003.2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer D.S. 2nd edn. Longman; London, UK: 1981. Introduction to quantitative genetics. [Google Scholar]
- Grift R.E, Rijnsdorp A.D, Barot S, Heino M, Dieckmann U. Fisheries-induced trends in reaction norms for maturation in North Sea plaice. Mar. Ecol. Prog. Ser. 2003;257:247–257. [Google Scholar]
- Hanson J.M, Chouinard G.A. Evidence that size-selective mortality affects growth of Atlantic cod (Gadus morhua L.) in the southern Gulf of St Lawrence. J. Fish Biol. 1992;41:31–41. doi:10.1111/j.1095-8649.1992.tb03168.x [Google Scholar]
- Hanson J.M, Chouinard G.A. Diet of Atlantic cod in the southern Gulf of St Lawrence as an index of ecosystem change, 1959–2000. J. Fish Biol. 2002;60:902–922. [Google Scholar]
- Heino M. Management of evolving fish stocks. Can. J. Fish. Aquat. Sci. 1998;55:1971–1982. doi:10.1139/cjfas-55-8-1971 [Google Scholar]
- Hutchings J.A. Collapse and recovery of marine fishes. Nature. 2000;406:882–885. doi: 10.1038/35022565. doi:10.1038/35022565 [DOI] [PubMed] [Google Scholar]
- Hutchings J.A. Life history consequences of overexploitation to population recovery in Northwest Atlantic cod. Can. J. Fish. Aquat. Sci. 2005;62:824–832. doi:10.1139/f05-081 [Google Scholar]
- Hutchings J.A, Reynolds J.D. Marine fish population collapses: consequences for recovery and extinction risk. BioScience. 2004;54:297–309. doi:10.1641/0006-3568(2004)054[0297:MFPCCF]2.0.CO;2 [Google Scholar]
- Koskinen M.T, Haugen T.O, Primmer C.R. Contemporary fisherian life-history evolution in small salmonid populations. Nature. 2002;419:826–830. doi: 10.1038/nature01029. doi:10.1038/nature01029 [DOI] [PubMed] [Google Scholar]
- Lankford T.E, Billerbeck J.M, Conover D.O. Evolution of intrinsic growth and energy acquisition rates II. Trade-offs with vulnerability to predation in Menidia menidia. Evolution. 2001;55:1873–1881. doi: 10.1111/j.0014-3820.2001.tb00836.x. doi:10.1554/0014-3820(2001)055[1873:EOIGAE]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Law R. Fishing, selection and phenotypic evolution. ICES J. Mar. Sci. 2000;57:659–668. doi:10.1006/jmsc.2000.0731 [Google Scholar]
- Law R, Grey D.R. Evolution of yields from populations with age-specific cropping. Evol. Ecol. 1989;3:343–359. doi:10.1007/BF02285264 [Google Scholar]
- MathSoft Inc. MathSoft, Inc; Seattle, WA: 1999. S-Plus 2000: guide to statistics. [Google Scholar]
- Merilä J, Sheldon B.C. Genetic architecture of fitness and nonfitness traits: empirical patterns and development of ideas. Heredity. 1999;83:103–109. doi: 10.1046/j.1365-2540.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- Morita K, Fukuwaka M. Does size matter most? The effect of growth history on probabilistic reaction norm for salmon maturation. Evolution. 2006;60:1516–1521. doi:10.1554/06-007.1 [PubMed] [Google Scholar]
- Mousseau T.A, Roff D.A. Natural selection and the heritability of fitness components. Heredity. 1987;59:181–197. doi: 10.1038/hdy.1987.113. [DOI] [PubMed] [Google Scholar]
- Olsen E.M, Heino M, Lilly G.R, Morgan M.J, Brattey J, Ernande B, Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. doi:10.1038/nature02430 [DOI] [PubMed] [Google Scholar]
- Reznick D.N, Ghalambor C.K. Can commercial fisheries cause evolution? Answers from guppies (Poecilia reticulata) Can. J. Fish Aquat. Sci. 2005;62:791–801. doi:10.1139/f05-079 [Google Scholar]
- Reznick D.N, Shaw F.H, Rodd F.H, Shaw R.G. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata) Science. 1997;275:1934–1937. doi: 10.1126/science.275.5308.1934. doi:10.1126/science.275.5308.1934 [DOI] [PubMed] [Google Scholar]
- Rijnsdorp A.D. Fisheries as a large-scale experiment on life-history evolution: disentangling phenotypic and genetic effects in changes in maturation and reproduction of North Sea plaice, Pleuronectes platessa L. Oecologia. 1993;96:391–401. doi: 10.1007/BF00317510. doi:10.1007/BF00317510 [DOI] [PubMed] [Google Scholar]
- Shelton P.A, Sinclair A.F, Chouinard G.A, Mohn R, Duplisea D.E. Fishing under low productivity conditions is further delaying recovery of Northwest Atlantic cod (Gadus morhua) Can. J. Fish Aquat. Sci. 2006;63:235–238. doi:10.1139/f05-253 [Google Scholar]
- Sinclair A, Chouinard G, Swain D, Hébert R, Nielsen G, Hanson M, Currie L, Hurlbut T. Assessment of the fishery for southern Gulf of St. Lawrence cod: May, 1994. DFO Atl. Fish. Res. Doc. 1994;94/77:116. [Google Scholar]
- Sinclair A.F, Swain D.P, Hanson J.M. Measuring changes in the direction and magnitude of size-selective mortality in a commercial fish population. Can. J. Fish Aquat. Sci. 2002a;59:361–371. doi:10.1139/f02-015 [Google Scholar]
- Sinclair A.F, Swain D.P, Hanson J.M. Disentangling the effects of size-selective mortality, density, and temperature on length-at-age. Can. J. Fish Aquat. Sci. 2002b;59:372–382. doi:10.1139/f02-014 [Google Scholar]
- Stockwell C.A, Hendry A.P, Kinnison M.T. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 2003;18:94–101. doi:10.1016/S0169-5347(02)00044-7 [Google Scholar]
- Stokes K, Law R. Fishing as an evolutionary force. Mar. Ecol. Prog. Ser. 2000;208:307–309. [Google Scholar]
- Swain D.P, Kramer D.L. Annual variation in temperature selection by Atlantic cod Gadus morhua in the southern Gulf of St Lawrence, Canada, and its relation to population size. Mar. Ecol. Prog. Ser. 1995;116:11–23. [Google Scholar]
- Swain D.P, Sinclair A.F, Castonguay M, Chouinard G.A, Drinkwater K.F, Fanning L.P, Clark D.S. Density- versus temperature-dependent growth of Atlantic cod (Gadus morhua) in the Gulf of St Lawrence and on the Scotian Shelf. Fish. Res. 2003;59:327–341. doi:10.1016/S0165-7836(02)00027-9 [Google Scholar]
- Thompson J.N. Rapid evolution as an ecological process. Trends Ecol. Evol. 1998;13:329–332. doi: 10.1016/s0169-5347(98)01378-0. doi:10.1016/S0169-5347(98)01378-0 [DOI] [PubMed] [Google Scholar]
- Walsh M.R, Munch S.B, Chiba S, Conover D.O. Maladaptive changes in multiple traits caused by fishing: impediments to population recovery. Ecol. Lett. 2006;9:142–148. doi: 10.1111/j.1461-0248.2005.00858.x. doi:10.1111/j.1461-0248.2005.00858.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Estimated selection differentials (cm) for length at age 4 yr for the spawning stocks producing the 1977 ? 1997 cohorts of Atlantic cod (Gadus morhua) in the southern Gulf of St. Lawrence (presented in Figure 2)