Abstract
Populations of many species are dramatically declining worldwide, but the causal mechanism remains debated among different human-related threats. Coping with this uncertainty is critical to several issues about the conservation and future of biodiversity, but remains challenging due to difficulties associated with the experimental manipulation and/or isolation of the effects of such threats under field conditions. Using controlled microcosm populations, we quantified the individual and combined effects of environmental warming, overexploitation and habitat fragmentation on population persistence. Individually, each of these threats produced similar and significant population declines, which were accelerated to different degrees depending upon particular interactions. The interaction between habitat fragmentation and harvesting generated an additive decline in population size. However, both of these threats reduced population resistance causing synergistic declines in populations also facing environmental warming. Declines in population size were up to 50 times faster when all threats acted together. These results indicate that species may be facing risks of extinction higher than those anticipated from single threat analyses and suggest that all threats should be mitigated simultaneously, if current biodiversity declines are to be reversed.
Keywords: population persistence, extinction risk, human-caused threats, climate change, overfishing, habitat loss
1. Introduction
Increase in the loss of species due to human-related threats is a major concern in modern ecology and conservation (Myers 1995; Pimm et al. 1996; Chapin et al. 2000; Pimm & Rave 2000; Sala et al. 2000; Novacek & Cleland 2001; Jenkins 2003). The decline of wild populations, which leads to species extinctions and consequently to reductions in overall biodiversity, has been mainly attributed to the effects of habitat fragmentation, overexploitation and global warming (see reviews by Myers 1995; Chapin et al. 2000; Sala et al. 2000; Novacek & Cleland 2001; Jenkins 2003; Parmesan & Yohe 2003). The specific effects and interactions of these threats remain, however, controversial because all threats do provide rational mechanisms to explain population declines; threats usually occur simultaneously and as yet there has been a lack of robust studies fully isolating their individual and combined effects (e.g. Myers 1995; Novacek & Cleland 2001; Jenkins 2003; Schiermeier 2003; Aronson et al. 2004; Buckley & Roughgarden 2004; Worm & Myers 2004; Grigg et al. 2005). Such uncertainty is regarded as one of the most important challenges in modern ecology (Myers 1995; Chapin et al. 2000; Sala et al. 2000; Novacek & Cleland 2001) and it is a major limitation in the construction of future projections on biodiversity change (Myers 1995; Sala et al. 2000), generation of effective conservation strategies (Schiermeier 2003; Worm & Myers 2004; Grigg et al. 2005) and quantification of extinction risks (Myers 1995; Sala et al. 2000; Novacek & Cleland 2001).
The potential mechanisms by which these threats are likely to affect species are relatively well acknowledged. Habitat fragmentation, for instance, increases the distance between populated habitats, which, in turn, reduces population connectivity and replenishment through immigration (Hanski 1998; Debinski & Holt 2000). The inflow of immigrants directly influences population dynamics and by introducing new genotypes it increases resistance and the speed of recovery (i.e. resilience) following perturbations. The inflow of immigrants also reduces the effects of inbreeding depletion, expands the geographical ranges of species, sustains populations that could not be maintained through self-recruitment and affects the probability of stochastic local extinctions (Hanski 1998; Debinski & Holt 2000; Cloberg et al. 2001; Mora & Sale 2002). In contrast, overexploitation removes members from the stock population beyond natural levels of replenishment causing populations to decline and indirectly reducing genetic diversity and the ability to adapt to other threats (Botsford et al. 1997; Jackson et al. 2001; Myers & Worn 2002; Pauly et al. 2002, 2003). Likewise, global warming is likely to reduce population size by causing mortality of non-resistant genotypes or by upsetting thermo-sensitive processes, such as reproduction (O'Brien et al. 2000; Mora & Ospina 2001, 2002; Stenseth et al. 2002; Walther et al. 2002; Parmesan & Yohe 2003). Alternatively, population density could be reduced to balance the higher energetic demands of maintaining metabolism in warmed environments (Poertner et al. 2001; Allen et al. 2002). Warming is also likely to displace optimum habitats, which may or may not be colonized depending upon species' capabilities to migrate (Warren et al. 2001). In the marine realm, warming is also likely to affect other environmental variables, such as rainfall and salinity, marine currents and productivity, all of which may affect populations in different ways and certainly complicates identifying the mechanism that causes populations to change under warming regimes. Unfortunately, while rational explanations exist for the mechanisms by which habitat fragmentation, overexploitation and warming affect biodiversity, identifying their actual effects in natural conditions has been challenging, because field experiments (to quantify and isolate their effects) are constrained by the spatial scales at which these threats operate and because, in most cases, these threats are occurring simultaneously.
A promising approach to reveal the causal link between environmental threats and biodiversity change is the use of microcosm experiments. Microcosms reduce ecosystem complexity allowing the isolation and test of specific factors (Huston 1999; Jessup et al. 2004). They also minimize confounding factors that plague observational studies, allow a high degree of experimental control, replication and accuracy, and using organisms with short generations, they provide relatively fast results even about processes that could be impossible to measure in nature during the span of a human life (Jessup et al. 2004). Microcosms have been useful in the understanding of many functional relationships that include environmental variables, fluctuating environments, ecological interactions, life-history traits, population dynamics, biodiversity, ecosystem functioning and evolution (e.g. Gause 1934; Hairston et al. 1968; Davies et al. 1998; McGrady-Steed et al. 1998; Petchey et al. 1999; Kassen et al. 2000; Fukami & Morin 2003; Fryxell et al. 2005; Kussell & Leibler 2005; Smith et al. 2005, reviewed by Jessup et al. 2004). On the downside, however, microcosms minimize ecosystem complexity and the multidimensionality of natural conditions. This characteristic has curiously been argued as the strength (Drenner & Mazumber 1999; Huston 1999; Jessup et al. 2004) and weakness (Carpenter 1999) of microcosms and of the entire field of experimental ecology (Huston 1999). However, reviews on the topic (i.e. Huston 1999; Jessup et al. 2004) have suggested that microcosms are a necessary step towards the understanding of complex ecological processes. Field experiments to assess factors, such as for instance the effect of harvesting, habitat fragmentation and warming at the scale that these occur are simply not feasible because the number of other environmental factors and variety of species is so great that an ‘empirical model of (such system) is both conceptually and logistically intractable’ (Huston 1999). The characteristics of microcosms and their broad use support their importance for revealing the causal link between human threats and biodiversity decline, particularly, when other approaches are scarce and when the current loss of biodiversity urgently calls for answers to mitigate deterring factors. Here, we used this tool to quantify the individual and combined effects of habitat fragmentation, overexploitation and environmental warming on population persistence.
2. Material and methods
(a) Study species
We used the rotifer species, Brachionus plicatilis, as the subject for this study. This species was chosen because it is easy to culture in captivity, its populations can stabilize at constant conditions (King 1967) and without expensive equipment cultures remain free of contamination. Contamination can be a problem with smaller organisms and may introduce confounding factors. This species is also highly prolific and can alternate reproductive strategies, which maximize its recovery from reductions in population size and enhance its levels of adaptation to selective forces (e.g. Yoshida et al. 2003). This suggests that the population responses of such organisms, to the factors analysed, are a very conservative measure of the potential effect that these factors may have on less resilient species.
(b) Experimental populations
We first generated a stock supply of the rotifer species. The stock was kept in three 20 l containers, which were placed in a room at 25°C and 12 h light cycle. The stock was fed approximately every 12 h with a constant supply of approximately 230 000 cells of Nannochloropsis sp. (Instant Algae) per millilitre of stock. This led to a constant population size of 60 rotifers per millilitre after two weeks, which was maintained throughout the experiment. Four weeks after the stock was set up, two of the stock containers were mixed and used to fill 300 transparent 50 ml centrifuge vials, which became the experimental microcosm populations. Throughout the experiment, microcosms and the remaining stock container were fed with the same food ratio and kept under the same light cycle. Vials and stock containers were constantly aerated and approximately 50% of their water was replaced every other day. To minimize the risk of contamination, the water used was filtered with a 1 μm filter and irradiated with UV light.
(c) Experimental design
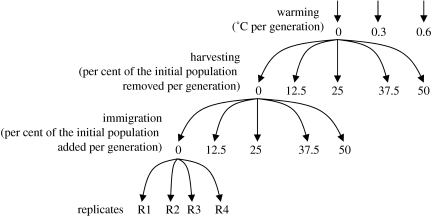
Our factorial experiment consisted of 300 replicate microcosm populations, initially at equilibrium, which were exposed to the effects of warming, harvesting and reductions in input immigrants (see scheme in figure 1). We used reductions in immigration as our surrogate for increases in habitat fragmentation. Our rationale is that habitat fragmentation increases isolation among populated patches, thereby reducing the amount of immigrants replenishing local populations (after Hanski 1998). It is important to clarify that, here, we are only dealing with the effect of the isolation produced by habitat fragmentation. Habitat fragmentation results from habitat loss, which may reduce the size of local populations if habitable area influences carrying capacity. This latter effect was not considered in the present study to avoid increases in the complexity of the experiment and risks of contamination associated with large experiments. Note that adding one more treatment with five levels to this experiment would have increased our number of microcosms to 1500. In this experiment, we considered as controls the populations that were exposed to no warming, no harvesting and had the highest inputs of immigrants. The highest level of immigration was chosen as the control for habitat fragmentation, because the opposite was to use total deprivation of immigration, which is the actual effect of this simulated threat.
Figure 1.
Diagram of the experimental design. The experiment consisted of three treatments for warming, five treatments for harvesting and five treatments for immigration; each treatment with four replicate microcosm populations (300 microcosm populations were deployed in total). For display purposes, arrows are shown to depict one treatment for each threat.
All microcosm populations (i.e. vials) were submerged into larger transparent aquaria to allow better control of water temperature (i.e. 12 aquaria, each with 25 vials). Each aquarium was equipped with an electronic temperature control (Autonics TZ4S), a temperature sensor (Autonics PT100) and two 300 W heaters. Using this set-up, water temperature was controlled with 0.1°C accuracy. Each of the microcosm populations, in each of the 12 aquaria, was exposed to one of the five levels of harvesting and immigration ranging from 0 to 50% removal or addition of the individuals in the initial population (figure 1). The maximum levels of harvesting and immigration were chosen based on reports about the levels of current exploitation and immigration to wild populations. Four aquaria were maintained at a constant temperature (i.e. 25°C), whereas the other eight were warmed to 33°C at a gradual rate of either 1 or 2°C per week. Note that standardized to the generation time of this rotifer species, these rates are equivalent to about 0.3 or 0.6°C per generation. These heating rates scale reasonably with warming rates that long-lived organisms might experience, considering that global average temperatures may increase from 1.4 to 5.8°C over the next century (Houghton et al. 2001) and that regional variation can be more intense due to localized thermal phenomena, such as El Nino (Fedorov & Philander 2000). The individuals used to replenish the microcosm populations (i.e. for the immigration treatment) were obtained from the stock. These individuals were added directly to populations at constant temperatures or slowly warmed to the temperature of warming treatments to avoid mortality due to thermal shock. In total, four replicated microcosm populations were used for each level of harvesting, immigration and warming. Densities of the microcosm populations were measured when warmed aquaria reached 33°C and after eight weeks in the aquaria that were kept at constant temperature.
(d) Data analysis
Change in population density for each microcosm population was calculated proportional to the initial density and standardized to generation time as (((final density−initial density)/initial density)/number of rotifer generations). In the analysed species generation time averaged 2006 days (calculated in this study). Changes in population time density among the different treatments were analysed with a factorial generalized linear model.
3. Results
(a) Independent effect of threats
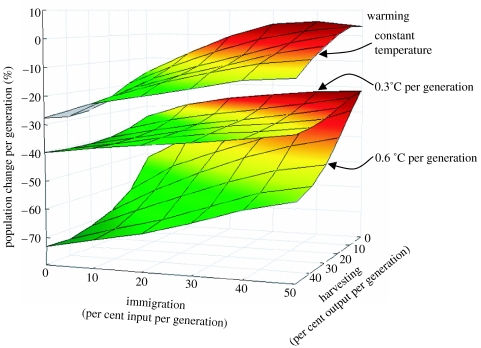
We found that independently all simulated threats caused significant population declines (p<0.000001; figure 2; table 1 in the electronic supplementary material). In contrast to control populations (i.e. populations with no warming, no harvesting and were replenished with immigrants), which changed only −1.4% per generation (±15 s.d.; figure 2; table 2 in the electronic supplementary material), all harvested populations declined below this value reaching a maximum decline of −13.4% per generation (±6 s.d.) at 50% harvesting per generation (figure 2; table 2 in the electronic supplementary material). The fact that a 50% population harvest per generation led to only a 13.4% decline in population size per generation indicates the fast population turnover in this rotifer species. In absolute terms, however, such a decline was 9.6 times faster than the change observed among non-harvested populations.
Figure 2.
Changes in population density, per generation, of populations exposed to harvesting and reductions in immigration, when facing constant and warming temperatures. The contours in the graph were based on averages of four replicated microcosms for each interaction of treatments. The raw data of this figure appear in table 2 in the electronic supplementary material.
Reductions in immigration showed negative effects on population size, when immigration was below 25% input per generation, reaching a maximum population decline of −16.6% per generation (±16.9 s.d.) when populations were totally deprived from immigration (figure 2; table 2 in the electronic supplementary material). This was a rate of decline 11.9 times faster than the change among replenished control populations (figure 2; table 2 in the electronic supplementary material). The fact that levels of immigration above 25% per generation did not cause burst increases in population density most probably reflects density-dependent control of population size and the stabilization of populations at carrying capacity.
Under control conditions of harvesting and immigration, both heating rates produced similar population declines (figure 2). This is an interesting result because one would have expected larger population declines at the quicker heating rate. However, it is important to note that the control conditions of this experiment included high inputs of immigrants. It is therefore most probable that the individuals counted in the treatments at both heating rates were immigrants. This ‘artefact’ has previously been observed in microcosm experiments and has been used to point the existence of sink populations (i.e. populations located in areas of extreme conditions, where self-replenishment is low to none and populations are maintained almost solely by immigration; e.g. Davies et al. 1998). When populations were deprived of immigration, population size at the quick heating rate declined 1.4 times faster than populations facing slower environmental warming (figure 2; table 2 in the electronic supplementary material). Fast warming caused population declines of −17.5% (±8 s.d.) per generation or a decline 11.8 times faster than control non-warmed populations.
(b) Pairwise effect of threats
The interaction between immigration and warming was significant (p<0.004; table 1 in the electronic supplementary material). Although all populations showed negative growths when facing warming (figure 2), deprivation of immigration accelerated such declines up to −50.8% (±20 s.d.) per generation under fast warming. This decline was 36.2 times faster than the change observed among control populations (figure 2; table 2 in the electronic supplementary material). Harvesting also showed a significant interaction with warming (p<0.05; table 1 in the electronic supplementary material). Population declines were accelerated up to −51.3% (±12.9 s.d.) per generation, when populations experienced high levels of harvesting and fast warming. This was a decline 36.6 times faster than the change observed among control non-harvested populations at constant temperatures (figure 2; table 2 in the electronic supplementary material). In general, rapid warming synergized the negative effects of harvesting and immigration deprivation (compare population declines at slow and fast warming rates in figure 2). Finally, the interaction between harvesting and immigration was non-significant (p=0.71). Populations that were intensely harvested and also deprived from immigration, in control conditions (i.e. no warming), declined −25.7% (±7.5 s.d.) or 18.5 times faster than control non-harvested and replenished populations (figure 2; table 2 in the electronic supplementary material). This net decline was about the same as adding the independent effect of both threats.
(c) Effect of all threats combined
The interaction among harvesting, immigration and warming was non-significant (p=0.19; table 1 in the electronic supplementary material). Although this third-order interaction did not cause accelerated declines in population size, the net effect of all the three factors acting together led to the largest declines in population size observed in this experiment (figure 2). Populations that were harvested and deprived from immigration and were in fast-warming environments declined −71.7% per generation (±11.7 s.d.) or a decline 51.6 times faster than control non-harvested and replenished populations at constant temperatures (figure 2).
4. Discussion
Increases in the rate of harvesting of wild species, destruction of their habitats and warming of their environment poses a major threat on their persistence (Myers 1995; Chapin et al. 2000; Sala et al. 2000; Jackson et al. 2001; Novacek & Cleland 2001; Jenkins 2003; Parmesan & Yohe 2003). The simultaneous occurrence of such threats has generated a major challenge to unravel their independent and combined effects through field experimentation, which, in turn, has generated uncertainty and strong controversies, but more importantly has precluded the development of earnest mitigation policies (e.g. Myers 1995; Novacek & Cleland 2001; Jenkins 2003; Schiermeier 2003; Aronson et al. 2004; Buckley & Roughgarden 2004; Worm & Myers 2004; Grigg et al. 2005). Using a microcosm experiment (see §1 for general description of pros and cons), we showed that independently each of these threats caused similar and significant population decays. As we mentioned in §1, different mechanisms can be involved in such declines. In threats that cause direct mortality, such as harvesting and warming, the natality may not balance the mortality losses, thereby leading to negative population growth. Other mechanisms may involve the effects of drift or perhaps depensation (i.e. reductions in per capita reproductive success at low population levels; e.g. Myers et al. 1995) in small depleted populations, inbreeding depletion on populations deprived from immigration and limited genetic diversity for adaptation to selective threats, such as warming. Regardless of the mechanism, it is clear that all threats do cause negative population growth, and therefore, are capable of driving populations to extinction.
In regards to the effects of warming, it is important to note that our results indicate that faster warming per generation, after removing the effect of immigration, led to faster population declines when compared with slower warming. These results highlight the importance of generation time in enhancing adaptation to selective forces and in explaining why some species have declined in step with global warming while others have not (e.g. Parmesan & Yohe 2003). These results also support the contention that species with long generation times are more prone to the effects of warming while highlighting the need to reduce the speed of the current warming trend (Stenseth et al. 2002; Walther et al. 2002; Parmesan & Yohe 2003). The overall decline of populations facing any warming also highlights the sensitivity of ecological systems to increases in temperature (Poertner et al. 2001) and suggests that environmental heating itself is capable of causing negative effects on populations independent of other environmental factors that may change in relation to warming (e.g. rainfall, currents, productivity, etc.). A caution with regard to our results is that, given the environmental gradients in nature, warming can transform habitats either from suitable to unsuitable or vice versa, and so may have negative or positive effects on population density. In our study, population size declined with warming, suggesting that our results apply to cases where warming reduces habitat suitability.
Quantifying the simultaneous effect of human-related threats is one of the major challenges in modern ecology and one of the main worries about the future of biodiversity (e.g. Myers 1995; Chapin et al. 2000; Sala et al. 2000; Novacek & Cleland 2001). Our study provides an insight into the nature and magnitude of such interactions. We found that the interaction between harvesting and reductions in the input of immigration, which results from isolation of habitats due to their fragmentation, was additive (i.e. they did not show a significant interaction). We presume that this may occur because both of these factors have a similar net effect on population size through the input and output of individuals. However, the reductions in population size due to these two factors caused synergistic declines in populations also facing warming. It is probable that the reductions in population size due to either harvesting or deprivation of immigration are accompanied with a loss of genetic diversity, which may impair population resilience to threats like warming. Importantly, there are empirical data showing accelerated decays of fragmented (Warren et al. 2001) and harvested (Finney et al. 2000; O'Brien et al. 2000) populations facing warming. This suggests that habitat fragmentation and harvesting do reduce population resistance to threats such as warming, that the experimental synergies found here do occur in nature and that species could be under higher risks of extinction than those anticipated from single threat analyses. As a first experimental insight into the effects of harvesting, habitat fragmentation and warming on population persistence, our study suggests that all threats are equally capable of causing deleterious effects and that they all have to be simultaneously reduced, if their synergies are to be avoided and if the loss of species is to be reversed.
Acknowledgments
We thank C. Neho, A. Cozens, M. Birch, J. Evans, T. Wustenberg and B. Dobson for collaboration with the experiment. K. Gaston, B. Worm, X. Atalah, J. McPherson and R. Ford provided valuable comments on the manuscript. Funding was provided by the Leigh Marine Laboratory and the Sloan Foundation Census of Marine Life through Future of Marine Animal Populations Project.
Supplementary Material
The file includes two tables with the results of the ANOVA and different statistics about the raw data of the experiment
References
- Allen A.P, Brown J.H, Gillooly J.F. Global biodiversity, biochemical kinetics, and the energy-equivalence rule. Science. 2002;297:1545–1548. doi: 10.1126/science.1072380. doi:10.1126/science.1072380 [DOI] [PubMed] [Google Scholar]
- Aronson R.B, et al. Causes of coral reef degradation. Science. 2004;302:1502–1504. doi: 10.1126/science.302.5650.1502b. doi:10.1126/science.302.5650.1502b [DOI] [PubMed] [Google Scholar]
- Botsford L.W, Castilla J.C, Peterson C.H. The management of fisheries and marine ecosystems. Science. 1997;277:509–515. doi:10.1126/science.277.5325.509 [Google Scholar]
- Buckley L.B, Roughgarden J. Effects of changes in climate and land use. Nature. 2004;430 doi: 10.1038/nature02717. doi:10.1038/nature02717 [DOI] [PubMed] [Google Scholar]
- Carpenter S.R. Microcosm experiments have limited relevance for community and ecosystem ecology. Ecology. 1999;80:1085–1088. doi:10.2307/177043 [Google Scholar]
- Chapin F.S, et al. Consequences of changing biodiversity. Nature. 2000;405:234–242. doi: 10.1038/35012241. doi:10.1038/35012241 [DOI] [PubMed] [Google Scholar]
- Cloberg J, Dhondt A.A, Nichols J.D. Oxford University Press; Oxford, UK: 2001. Dispersal. [Google Scholar]
- Davies A.J, Jenkinson L.S, Lawton J.H, Shorrrocks B, Wood S. Making mistakes when predicting shifts in species range in response to global warming. Nature. 1998;391:783–786. doi: 10.1038/35842. doi:10.1038/35842 [DOI] [PubMed] [Google Scholar]
- Debinski D.M, Holt R.D. A survey and overview of habitat fragmentation experiments. Conserv. Biol. 2000;14:342–355. doi:10.1046/j.1523-1739.2000.98081.x [Google Scholar]
- Drenner R.W, Mazumber A. Microcosm experiments have limited relevance for community and ecosystem ecology: comment. Ecology. 1999;80:1081–1085. doi:10.2307/177042 [Google Scholar]
- Fedorov A.V, Philander G. Is El Niño changing? Science. 2000;288:1997–2002. doi: 10.1126/science.288.5473.1997. doi:10.1126/science.288.5473.1997 [DOI] [PubMed] [Google Scholar]
- Finney B.P, Gregory-Eaves I, Sweetman J, Douglas M.S.V, Smol J. Impacts of climatic change and fishing on Pacific salmon abundance over the past 300 years. Science. 2000;290:795–799. doi: 10.1126/science.290.5492.795. doi:10.1126/science.290.5492.795 [DOI] [PubMed] [Google Scholar]
- Fryxell J.M, Smith I.M, Lynn D.H. Evaluation of alternate harvesting strategies using experimental microcosms. Oikos. 2005;111:143–149. doi:10.1111/j.0030-1299.2005.13840.x [Google Scholar]
- Fukami T, Morin P. Productivity–biodiversity relationships depend on the history of the community assembly. Nature. 2003;424:423–426. doi: 10.1038/nature01785. doi:10.1038/nature01785 [DOI] [PubMed] [Google Scholar]
- Gause G.F. Dover; New York, NY: 1934. The struggle for existence. [DOI] [PubMed] [Google Scholar]
- Grigg R.W, et al. Reassessing U.S. coral reefs. Science. 2005;308:1740–1742. doi: 10.1126/science.308.5729.1740c. doi:10.1126/science.308.5729.1740c [DOI] [PubMed] [Google Scholar]
- Hairston N.G, Allan J.D, Colwell R.K, Futuyma D.J, Howell J, Lubin M.D, Mathias J, Vandermeer J.H. The relationship between species diversity and stability: an experimental approach with protozoa and bacteria. Ecology. 1968;49:1091–1101. doi:10.2307/1934492 [Google Scholar]
- Hanski I. Metapopulation dysnamics. Nature. 1998;396:41–49. doi:10.1038/23876 [Google Scholar]
- Houghton J.T, Ding Y, Griggs D.J, Noguer M, van der Linden P.J, Xiaosu D, editors. Climate change: the scientific basis. Cambridge University Press; Cambridge, UK: 2001. [Google Scholar]
- Huston M.A. Microcosm experiments have limited relevance for community and ecosystem ecology: synthesis of comments. Ecology. 1999;80:1088–1089. doi:10.2307/177044 [Google Scholar]
- Jackson J.B.C, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. doi:10.1126/science.1059199 [DOI] [PubMed] [Google Scholar]
- Jenkins M. Prospects for biodiversity. Science. 2003;302:1175–1177. doi: 10.1126/science.1088666. doi:10.1126/science.1088666 [DOI] [PubMed] [Google Scholar]
- Jessup C.M, Kassen R, Forde S.E, Kerr B, Buckling A, Rainey P.B, Bohannan B.J.M. Big questions, small worlds: microbial model systems in ecology. Trends Ecol. Evol. 2004;19:189–197. doi: 10.1016/j.tree.2004.01.008. doi:10.1016/j.tree.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Kassen R, Buckling A, Bell G, Rainey P.B. Diversity peaks at intermediate productivity in a laboratory microcosm. Nature. 2000;406:508–512. doi: 10.1038/35020060. doi:10.1038/35020060 [DOI] [PubMed] [Google Scholar]
- King C.E. Food, age, and dynamics of a laboratory population of rotifers. Ecology. 1967;48:111–128. doi:10.2307/1933423 [Google Scholar]
- Kussell E, Leibler S. Phenotypic diversity, population growth, and informatics in fluctuating environments. Science. 2005;309:2075–2078. doi: 10.1126/science.1114383. doi:10.1126/science.1114383 [DOI] [PubMed] [Google Scholar]
- McGrady-Steed J, Harris P.M, Morin P. Biodiversity regulates ecosystem predictability. Nature. 1998;390:162–165. [Google Scholar]
- Mora C, Ospina F. Thermal tolerance and potential impact of sea warming on reef fishes from Gorgona island (eastern Pacific Ocean) Mar. Biol. 2001;139:765–769. doi:10.1007/s002270100626 [Google Scholar]
- Mora C, Ospina F. Experimental effects of cold, La Nina temperatures in the survival of reef fishes from Gorgona Island (eastern Pacific Ocean) Mar. Biol. 2002;141:789–793. doi:10.1007/s00227-002-0862-1 [Google Scholar]
- Mora C, Sale P.F. Are populations of coral reef fishes open or closed? Trends Ecol. Evol. 2002;17:422–428. doi:10.1016/S0169-5347(02)02584-3 [Google Scholar]
- Myers N. Environmental unknowns. Science. 1995;269:358–360. doi: 10.1126/science.269.5222.358. doi:10.1126/science.269.5222.358 [DOI] [PubMed] [Google Scholar]
- Myers R.A, Worn B. Rapid worldwide depletion of predatory fish communities. Nature. 2002;423:280–283. doi: 10.1038/nature01610. doi:10.1038/nature01610 [DOI] [PubMed] [Google Scholar]
- Myers R.A, Barrowman N.J, Hutchings J.A, Rosenberg A.A. Population dynamics of exploited fish stocks at low population levels. Science. 1995;269:1106–1108. doi: 10.1126/science.269.5227.1106. doi:10.1126/science.269.5227.1106 [DOI] [PubMed] [Google Scholar]
- Novacek M, Cleland E.E. The current biodiversity extinction event: scenarios for mitigation and recovery. Proc. Natl Acad. Sci. USA. 2001;98:5466–5470. doi: 10.1073/pnas.091093698. doi:10.1073/pnas.091093698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C.M, Fox C.J, Planque B, Casey J. Climate variability and North Sea cod. Nature. 2000;404:142. doi: 10.1038/35004654. doi:10.1038/35004654 [DOI] [PubMed] [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. doi:10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- Pauly D, Christensen V, Guénette S, Pitcher T.J, Sumaila U.R, Walters C.J, Watson R, Zeller D. Towards sustainability in world's fisheries. Nature. 2002;418:689–695. doi: 10.1038/nature01017. doi:10.1038/nature01017 [DOI] [PubMed] [Google Scholar]
- Pauly D, Alder J, Bennett E, Christensen V, Tyedmers P, Watson R. The future for fisheries. Science. 2003;302:1359–1361. doi: 10.1126/science.1088667. doi:10.1126/science.1088667 [DOI] [PubMed] [Google Scholar]
- Petchey O.L, McPhearson P.T, Casey T.M, Morin P.J. Environmental warming alters food-web structure and ecosystem function. Nature. 1999;402:69–72. doi:10.1038/47023 [Google Scholar]
- Pimm S.L, Rave P. Extinctions by numbers. Nature. 2000;403:843–845. doi: 10.1038/35002708. doi:10.1038/35002708 [DOI] [PubMed] [Google Scholar]
- Pimm S.L, Russell G.J, Gittleman J.L, Brooks T.M. The future of biodiversity. Science. 1996;269:347–350. doi: 10.1126/science.269.5222.347. doi:10.1126/science.269.5222.347 [DOI] [PubMed] [Google Scholar]
- Poertner H.O, et al. Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: developing a hypothesis for cause and effect relationships in Atlantic cod Gadus morhua and common eelpout Zoarces viviparus. Cont. Shelf Res. 2001;21:1975–1997. doi:10.1016/S0278-4343(01)00038-3 [Google Scholar]
- Sala O.E, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. doi:10.1126/science.287.5459.1770 [DOI] [PubMed] [Google Scholar]
- Schiermeier Q. Climate findings let fishermen off the hook. Nature. 2003;428:4. doi: 10.1038/428004a. doi:10.1038/428004a [DOI] [PubMed] [Google Scholar]
- Smith V.H, Foster B.L, Grover J.P, Holt R.D, Leibold M.A, DeNoyelles F., Jr Phytoplankton species richness scales consistently from laboratory microcosms to the world's oceans. Proc. Natl Acad. Sci. USA. 2005;102:4393–4396. doi: 10.1073/pnas.0500094102. doi:10.1073/pnas.0500094102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth N.C, Mysterud A, Ottersen G, Hurrell J.W, Chan K.-S, Lima M. Ecological effects of climate fluctuations. Science. 2002;297:1292–1296. doi: 10.1126/science.1071281. doi:10.1126/science.1071281 [DOI] [PubMed] [Google Scholar]
- Walther G, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.-M, Hoegh-Guldberg O, Bairlein F. Ecological response to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. doi:10.1038/416389a [DOI] [PubMed] [Google Scholar]
- Warren M.S, et al. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature. 2001;414:65–69. doi: 10.1038/35102054. doi:10.1038/35102054 [DOI] [PubMed] [Google Scholar]
- Worm B, Myers R.A. Managing fisheries in a changing climate. Nature. 2004;429:15. doi: 10.1038/429015a. doi:10.1038/429015a [DOI] [PubMed] [Google Scholar]
- Yoshida T, Jone L.E, Ellner S.P, Fussmann G.F, Hairston N.G. Rapid evolution drives ecological dynamics in a predator–prey interaction. Nature. 2003;424:303–306. doi: 10.1038/nature01767. doi:10.1038/nature01767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The file includes two tables with the results of the ANOVA and different statistics about the raw data of the experiment