Abstract
Rats sweep their facial whiskers back and forth to generate tactile sensory information through contact with environmental structure. The neural processes operating on the signals arising from these whisker contacts are widely studied as a model of sensing in general, even though detailed knowledge of the natural circumstances under which such signals are generated is lacking. We used digital video tracking and wireless recording of mystacial electromyogram signals to assess the effects of whisker–object contact on whisking in freely moving animals exploring simple environments. Our results show that contact leads to reduced protraction (forward whisker motion) on the side of the animal ipsilateral to an obstruction and increased protraction on the contralateral side. Reduced ipsilateral protraction occurs rapidly and in the same whisk cycle as the initial contact. We conclude that whisker movements are actively controlled so as to increase the likelihood of environmental contacts while constraining such interactions to involve a gentle touch. That whisking pattern generation is under strong feedback control has important implications for understanding the nature of the signals reaching upstream neural processes.
Keywords: active sensing, vibrissae, rat whisking, motor pattern generation
1. Introduction
‘Active sensing’ involves controlling the position and orientation of the sensory apparatus so as to enhance the organism's capacity to obtain behaviourally relevant information (Gibson 1962; Aloimonos et al. 1988; Ballard 1991; Chapman 1994; Lungarella et al. 2005). The rat whisker system is widely seen as a paradigmatic example of such an active sense system (Hartmann 2001; Szwed et al. 2003; Derdikman et al. 2006; Kleinfeld et al. 2006), even though our understanding of the strategies that guide whisker movements during natural behaviour is limited, in part due to the difficulty of accurately observing whisker positions in freely moving animals. The neural processes that operate on the signals arising from whisker contact with the environment are also widely studied as a useful model of mammalian sensory processing (e.g. Harris et al. 1999; Ahissar & Arieli 2001; Dyck 2005; Feldman & Brecht 2005; Bruno & Sakmann 2006; Kleinfeld et al. 2006). The correct interpretation of contact-induced activity in neural pathways will depend, however, on understanding how whisker movements are regulated; both because evoked activity in the somatosensory system differs according to whether a whisker is actively moved against an object or passively deflected (Derdikman et al. 2006; Ferezou et al. 2006), and because active control strategies may constrain the types of whisker deflections to which upstream processes are exposed. The study of whisker control in behaving animals is therefore an essential part of the ongoing investigation of this important model sensory system.
During exploratory behaviour, the large facial whiskers (‘macrovibrissae’) of the rat are swept back and forth such that the tips follow curved trajectories sampling the space surrounding the animal's head and snout (Vincent 1912; Gustafson & Felbain-Keramidas 1977; Carvell & Simons 1990; Brecht et al. 1997; Bermejo et al. 2002; Kleinfeld et al. 2006). This ‘whisking’ is performed in bouts of one to many individual ‘whisks’. Whisker movements are driven by pattern generators that in unobstructed, or ‘free’, whisking appear to operate independently of direct sensory feedback (Gao et al. 2001; Berg & Kleinfeld 2003a). Studies of free whisking in the head-fixed animal further report a preponderance of bilateral symmetry and synchrony, and stereotypical kinematics within, and to a lesser extent between, bouts (Gao et al. 2001; Bermejo et al. 2002; Berg & Kleinfeld 2003a; Sachdev et al. 2003). Individual primary afferents innervating each whisker follicle respond reliably to step deflections of their associated whisker, in a way that has been repeatedly characterized in animals under various levels of anaesthesia (Gibson & Welker 1983a,b; Lichtenstein et al. 1990; Shoykhet et al. 2000; Jones et al. 2004). It is tempting, then, to think that the signals generated during natural whisker movement can be reliably inferred from those derived during unmodulated whisking behaviour such as that observed during artificially induced invariant whisking against an obstacle (Szwed et al 2003; Yu et al. 2006). Recent observations on unrestrained animals show, however, that the kinematics of whisking can vary considerably, but predictably, even on time-scales shorter than a single whisk (Towal & Hartmann 2006). This implies that the encoding of the environment generated at the sensory periphery could depend strongly on the nature of the whisking control strategy. In our own high-speed video recordings, we have noted that whisking in the freely moving animal is frequently directed towards nearby surfaces, with multiple whiskers making environmental contacts during a typical whisk cycle, even where the rat is proceeding across a flat, featureless floor (Prescott et al. 2005). These observations suggest that the study of whisking pattern generation during natural behaviour might produce different results from those obtained in studies in which whisking is largely or entirely unobstructed.
Recording whisking behaviour in freely moving rats is technically challenging; however, it is becoming possible to make detailed observations of whisking control in such circumstances (Hartmann et al 2000; Hartmann 2001; Knutsen et al. 2004; Prescott et al. 2005; Towal & Hartmann 2006). Here, we report two experiments which demonstrate that whisking pattern generation in unrestrained animals is strongly modulated by contact with the environment. In the first experiment, we use quantitative analysis of multiple short clips of high-speed video to show evidence of fast feedback modulation within a single whisk cycle. In the second experiment, we use longer recordings (equivalent to many thousands of whisk cycles) of whisker electromyogram (EMG) data, obtained alongside automated tracking of head movements, to show that feedback-induced asymmetry is a consistent feature of whisking behaviour in the presence of a unilateral obstruction such as a single nearby wall.
2. Results
(a) High-speed videography of contact-induced whisking modulation
Rat whiskers have a small diameter (typically less than 0.1 mm at the base) and peak instantaneous velocities that, during exploratory whisking, can exceed 1 ms−1. Obtaining useful observations of their movements therefore requires specialized recording equipment (Dyck 2005). Recent improvements in the spatial resolution of digital high-speed video enabled us to film free moving, untrained rats exploring simple environments and to record the whisking strategies used under these ethologically relevant conditions; the observed contact-invoked modulations are very striking. With apparent great reliability, unilateral obstruction of the whiskers rapidly suppresses ipsilateral protraction, while exciting contralateral protraction in a pronounced way during subsequent whisks (figure 1). To elucidate the time-scale of the suppression, we examined multiple high-speed video clips of rats making contact with a vertical surface, on a forward whisker (column 3 or higher), while proceeding across a featureless floor (see video 1 of electronic supplementary material for an example). For each clip, we tracked the movement of one rearward whisker (column 1 or 2) on each side of the snout so as to obtain times of whisker protraction onset and cessation, bilaterally and relative to the initial contact. In each case, the tracked whiskers did not touch the contacted surface at any time, so cessation of protraction was not due to physical obstruction of their movement. Results from an analysis of 22 clips are graphed in figure 2 and indicate that ipsilateral protraction ceased quickly and reliably with a mean time lag of 13 ms after contact (s.d. 7 ms). Contralateral protraction, by contrast, appeared to continue to a natural stopping point (mean time lag 29 ms, s.d. 20 ms) that was independent of the ipsilateral contact event. This difference was demonstrated statistically by comparison of protraction onset and cessation times. Specifically, protraction onset times were similar on both sides of the snout (Wilcoxon signed-rank test; Z=1.38, p=0.167, n=22), but cessation occurred earlier on the ipsilateral side than on the contralateral side (Z=3.41, p=0.001, n=22). Note that cessation of protraction on the ipsilateral side was immediately followed by retraction (see video 1 of electronic supplementary material), indicating that the effect of object contact on whisker movement is not purely mechanical, but involves modulation of the whisker drive system.
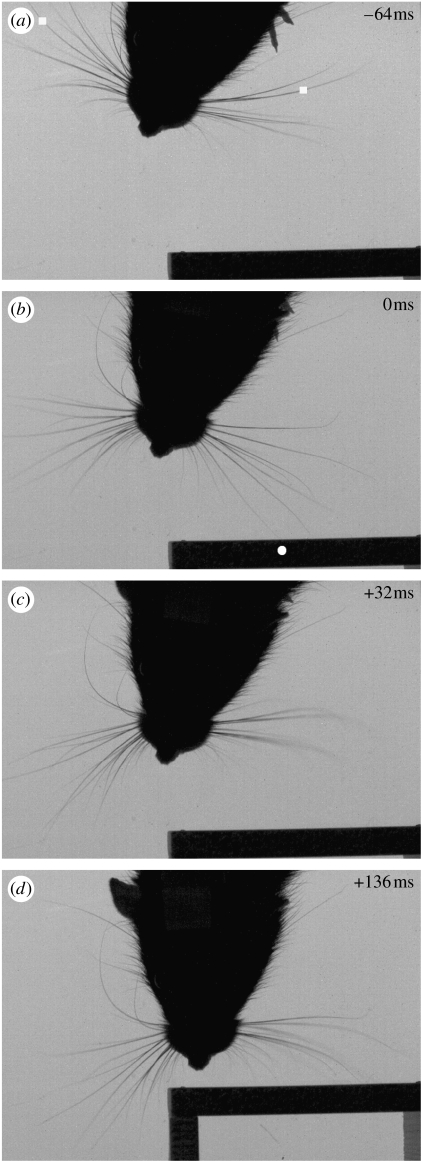
Figure 1.
High-speed video frames showing the effect of unilateral object contact (at t=0) on bilateral whisker protraction. (a) (t=−64 ms) Protraction commences approximately synchronously on both sides of the snout; the filled white squares show the tracked rear column whiskers. (b) (t=0 ms) A deflection occurs on a forward whisker, the filled white circle indicates the point of contact with the vertical surface. (c) (t=+32 ms) Protraction ends on the side contralateral to the contact, note that whiskers on the ipsilateral side are already partially retracted having ceased protraction at t=+12 ms. (d) (t=+136 ms) Contralateral whiskers reach maximum protraction in the whisk cycle subsequent to the initial contact (note that the interposed retraction is not shown). Protraction amplitude in this whisk is notably increased contralaterally, compared with whisks preceding contact, and reduced ipsilaterally such that the whiskers on that side are only gently deflected by the surface contact. Video 1 of electronic supplementary material shows the full whisk cycle bracketing the initial deflection.
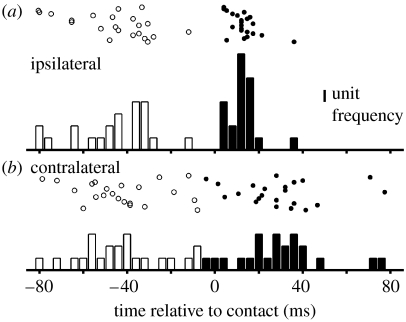
Figure 2.
(a) Ipsilateral and (b) Contralateral whisker protraction onset (open bars) and cessation (filled bars) relative to time of contact (t=0) of a forward whisker with an obstacle. Protraction onset times are similar on both sides of the snout, but cessation occurs earlier on the ipsilateral side than on the contralateral side and appears more closely tied to the time of contact (note the pronounced peak ipsilaterally approximately 13 ms after contact). Data are from 22 high-speed video clips recorded with nine different animals.
(b) Wireless electromyogram monitoring of whisking behaviour
While high-speed digital video is an effective tool for gathering detailed information over short time periods, we were inspired by the above results to quantify the effects of environmental contact on whisker control at longer time-scales which required different techniques. Rat mystacial EMG has been shown to provide a useful proxy for whisker protraction (Carvell et al. 1991; Berg & Kleinfeld 2003a; Sachdev et al. 2003; Cramer & Keller 2006); however, accessing the recording electrodes using trailing wires can restrict the freedom of movement of the animal and obscure the view of video recording equipment. To overcome these limitations, we have developed a purpose-built telemetry system that allows wireless recording of whisker EMG in unrestrained animals. For the current study, three rats were implanted bilaterally and whisker tracking in high-speed video recordings was used to confirm a positive correlation, on each side of the snout, between whisker protraction and EMG strength (figure 3 and video 2 of electronic supplementary material). Each animal was then allowed, on 4 or 5 separate days, to roam freely in a featureless glass-lidded, rectangular arena until it lost interest in exploring. During each session, which lasted between 5 and 30 min, EMG was continuously recorded and used to compute a slow-varying approximation of left and right whisking amplitudes. At the same time, automated tracking of normal digital video provided frame-by-frame estimates of the position and bearing of the rat relative to the arena walls (see figure 4a and video 3 of electronic supplementary material). Video and EMG data from each session were further processed to obtain sequences of frames at a sample rate of 8.33 Hz, which is towards the middle of the observed frequency range for rat exploratory whisking (5–15 Hz; Berg & Kleinfeld 2003a); thus, each frame sampled approximately one whisk cycle. From about 3 h of recordings, taken over 13 sessions, we obtained 26 040 such frames (approx. 52 min), in which an animal was at an identifiable location and displaying robust whisking activity. Rat whiskers do not generally exceed 100 mm in length (Brecht et al. 1997), accordingly we extracted two subsets of this data for further analysis: 3965 frames (approx. 8 min) in which the nose was within 25 mm of one wall and at least 100 mm from all other walls (the NEAR subset), and 5989 frames (approx. 12 min) in which the nose was at least 100 mm from all walls (the FAR subset). In the following, we report analyses combined across all three animals, detailed results for individuals are given in the supplementary information of electronic supplementary material.
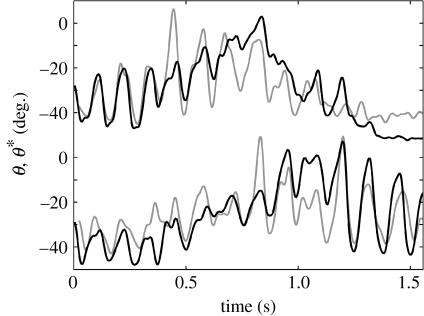
Figure 3.
Mystacial EMG as a proxy measure of whisking, example in one animal. Black trace is whisker angle (θ), determined by high-speed video tracking, of the left/right (top/bottom) whisker field (column 1); note separate y-axis origins for each trace. Grey trace (θ*) is an estimate of θ derived from left/right whisking EMG data. Increasing θ corresponds to whisker protraction for all traces.
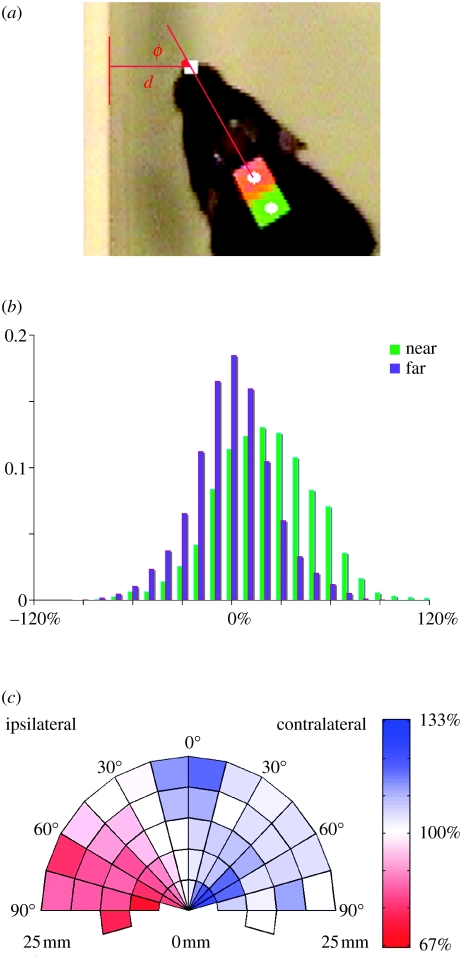
Figure 4.
Proximity to a wall induces a systematic bias in whisking asymmetry. (a) Distance (d) and bearing (ϕ) to a single nearby wall was determined by automated tracking of normal speed video, allowing the data to be partitioned into NEAR (d≤25 mm) and FAR (d≥100 mm) subsets, and for left and right whisking amplitudes to be classified as ipsilateral or contralateral. (b) Normalized frequency histograms of NEAR (green, n=3965) and FAR (purple, n=5989) whisking bias (contralateral–ipsilateral amplitude) as a percentage of average whisk strength. The distributions show a strong bias (mean 21.5%) in favour of contralateral whisking for NEAR only. Plots for individual animals are given in the electronic supplementary material. (c) Polar plot of ipsilateral (shown left of the midline) and contralateral (shown right of midline) mean whisking amplitude binned, for NEAR frames only, according to d and ϕ. For the purpose of this display, the nearby wall is always to the left with its bearing and distance mirrored across the midline (frames in which the wall was originally to the right have therefore been reflected in the midline). The colour scale (red–white–blue) shows increasing percentage of average whisk strength. Bins with a count of less than five frames are omitted (3955 frames displayed); included bins represent 5–541 frames each (median 92). The presence of a nearby wall leads to reduced ipsilateral and increased contralateral whisking. Ipsilateral reduction is most pronounced in the range 45–90°, where the rat is more ‘side-on’ to the obstruction. Where the wall lies directly in front (0–15°), but at some distance (d>15 mm), there is evidence of increased whisking bilaterally. Data for individual animals are given in the electronic supplementary material.
In the NEAR dataset, the difference (left–right) in whisking amplitude was found to be strongly inversely correlated (r=−0.64) with the bearing from the nose to the closest point on the nearby wall (positive on the left and negative on the right). In other words, and mirroring the briefer observations made with high-speed video, animals whisked more strongly on the right when a wall lay to the left and vice versa. Conversely, and as expected, in FAR, the bearing to the nearest wall was a poor predictor of left–right asymmetry (r=0.01). This difference in correlation was a robust effect found in each animal and every recording session (repeated measures ANOVA: F1=175.14, p=0.006, power=1.0). Based on the bearing to the nearest wall, the left and right amplitude data for all frames were then sorted into ipsilateral and contralateral classes. In NEAR, mean contralateral whisking amplitude was 110.8% (s.d. 22.1) of average whisk strength (mean amplitude across all data in both subsets), ipsilaterally 89.4% (s.d. 21.0)—a difference or whisking bias of 21.5% (29.4) (figure 4b). Again, there was a significant and consistent contrast with FAR in all animals and sessions (F1=24.68, p=0.038, power=0.72) with recordings in that subset showing no systematic deviation from symmetric whisking with respect to the nearest wall (contralateral 99.6% (19.3), ipsilateral 100.3% (19.8), bias −0.7% (24.4)). In NEAR, 38.1% of frames showed strong asymmetry equal to one-third or more of average whisk strength, almost all in the expected direction (contralateral stronger in 34.8%), whereas in FAR, this degree of asymmetry occurred in just 16.8% of frames.
Further analysis of the NEAR subset is shown in figure 4c and confirms the systematic nature of the environment-induced whisking asymmetry. This plot shows ipsilateral (shown left of the midline) and contralateral (right of midline) mean whisking amplitude binned according to both the distance and direction to the single nearby wall. The plot confirms that the presence of a nearby wall leads to reduced ipsilateral and increased contralateral whisking and further demonstrates that the ipsilateral reduction is most pronounced in the range 45–90°, where the rat is more ‘side-on’ to the obstruction. Where the wall lies directly in front (0–15°), but at some distance (d>15 mm), there is evidence of increased whisking bilaterally. Similar plots for individual animals are given in the electronic supplementary material.
Based on the insights gleaned from the above analysis, we formed a final, and more constrained, data subset, SIDE-ON, using the 1009 frames (approx. 2 min), sampled across all animals, in which the nose was within 10 mm of one wall and at least 100 mm from all other walls, and the bearing to the nearby wall was 30° or greater (i.e. the wall was very close by and more to one side than head-on). For this subset, we found a mean bias of 36% and r=−0.79 (compared with 21.5% and r=−0.64 for the full NEAR subset). Under these circumstances, contralateral amplitude also exceeded ipsilateral by an average ratio of 3 : 2, and by 2 : 1 or greater in 15% of frames. A typical situation that might generate such data is where the animal is moving slowly alongside a wall; in such circumstances, we have frequently observed very pronounced whisking asymmetry in our high-speed video recordings. An example of this type of behaviour is shown in figure 5 and is also illustrated in video 4 of electronic supplementary material.
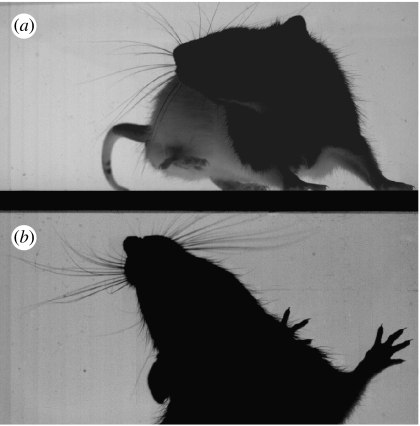
Figure 5.
High-speed video frame showing asymmetric whisking against a wall. (a) The image of the animal in the vertical plane obtained using a front-silvered mirror, positioned behind a glass wall, and slanted at an oblique angle with respect to the camera. (b) The view in horizontal plane, with the viewpoint of the camera aligned with the vertical surface of the wall. (Note that the dark strip across the centre of the frame therefore corresponds to the floor of the arena for (a) and the wall for (b).) The snapshot shows the moment of maximum protraction for a single whisk. Protraction is strongly asymmetric in a manner that appears to reduce bending of the whiskers on the side of the snout closest to the wall, while increasing the likelihood of surface contact by whiskers on the opposite side. An extract from the video clip from which this frame was taken is provided in video 4 of electronic supplementary material.
3. Discussion
Our results constitute the first quantitative demonstration of modulation of whisking pattern generation resulting from whisker contact with environmental structure. Our EMG data further demonstrate that this modulation is an active process presumably driven by sensory feedback to the vibrissal motor neurons for which there are multiple possible neural substrates (see below). Functionally, we suggest that the ipsilateral suppression aspect of whisker control will tend to constrain the dynamic range of contact events. The advantages of this are as follows. First, all the sensory apparatus of the whisker is concentrated at the base, and deflections of the whisker from its unforced shape will introduce noise onto measurements made there. Second, under this suppression, the sensory apparatus serving the whisker will experience a smaller range of inputs, allowing the same encoding system to provide better resolution without overloading. Additionally, departures from the stereotypical contacts generated by this scheme, such as contacts with moving objects, should be all the more noticeable. To complement the modulations prompted by ipsilateral contacts, we propose that the functional role of the excitation of contralateral protraction is to elicit more frequent contact between the whiskers and the environment. During unilateral contact, for instance, positive feedback will bring the contralateral whisker field to the front of the animal and towards the most probable location of environmental features already encountered ipsilaterally, increasing the rate of collection of information.
Given the above functional interpretation, we might also expect to see bilateral excitation of protraction given prior knowledge of items of interest located in front of the animal. This idea is supported by the analysis given in figure 4c, which shows increased protraction on both sides of the snout in the presence of walls 15 mm or more in front of the animal and perpendicular to its current bearing (i.e. head-on). This hypothesis is also consistent with data from Carvell & Simons (1990) who observed increased bilateral protraction towards an expected stimulus associated with reward, and from Sachdev et al. (2003) who observed increased ipsilateral protraction towards a similarly expected unilateral contact. Overall, our results indicate that asymmetry in whisking behaviour is more pronounced near walls; however, we did measure some asymmetry away from walls which might have been due to anticipation of head movements, as proposed by Towal & Hartmann (2006), or to unmeasured contacts with the floor or ceiling.
The above results can be summarized by saying that rat whisking uses two active control strategies. The first, which we term ‘minimal impingement’, seeks to limit the amount of bending that occurs in the whisker shaft on surface contact and thus the extent to which the whisker ‘impinges’ upon the environment. The second, which we term ‘maximal contact’, tries to orient the two halves of the whisker field so as to bring as many whiskers as possible to bear on surfaces or objects of interest. This active control of whisker actuation thus allows the rat to ‘home-in’ on interesting environment structure while ensuring that the resulting contacts are made using a gentle touch. Humans similarly use active strategies to control the position of the tactile sensory surfaces on the fingertips (Chapman 1994) and also regulate the pressure with which contacts are made according to the task in hand (Smith et al. 2002). Therefore, our findings may be relevant to the wider understanding of feedback control in the mammalian sense of touch.
While the control strategies described here can account for much of the variance in whisking bias in our behavioural data, it is worth noting that we have seen a number of departures from these principles in our high-speed video recordings. Specifically, when the rat is directing its snout towards an object that lies in front, an obstruction to one side does not appear to elicit the usual level of ipsilateral suppression. Likewise, contralateral excitation appears to be elicited less strongly, or not at all, on those occasions where the animal indicates disinterest by failing to orient to an encountered obstruction. A parsimonious explanation of these observations would be that both feedback control strategies occur primarily in relation to objects towards which the rat is directing its attention, which would imply a neural capacity to modulate or override mechanisms implementing the proposed feedback loops. It is also important to note that our untrained animals were expressing a particular class of exploratory behaviour—albeit one that we hold to have particular ethological relevance—and that animals trained to perform some other task (e.g. texture discrimination) might exhibit a modified control strategy.
Our analysis further indicates that the ipsilateral suppression has a relatively short time-scale (approx. 13 ms). Given that time is required for the musculature and mechanics to respond to motor neuron suppression, we suspect that the feedback responsible must be fairly direct. The probable source of this feedback is from the trigeminal primary afferent neurons, noted earlier, and in particular, from those cells that show a strong response to contact onset (e.g. Szwed et al. 2003). Bilateral projections from the trigeminal sensory complex to the facial motor nuclei that drive the intrinsic (protraction) muscles (lateral VII) have been identified (Dauvergne et al. 2002), including inputs arising from inhibitory cells (Li et al. 1997); however, a recent report suggests that ipsilateral whisker–sensory feedback in this loop is largely excitatory (Nguyen & Kleinfeld 2005). In light of this, and the involvement of higher centres implied by motivational effects, it seems probable that the neural substrate for whisking modulation due to environmental contact will involve sensorimotor loops at several anatomical levels (Kleinfeld et al. 1999; Prescott et al. 1999) including, potentially, those involving the basal ganglia (McHaffie et al. 2005) and the motor cortex (Kleinfeld et al. 2006).
We believe that feedback control of whisking pattern generation, on the time-scales observed here, will strongly influence the signals arising in the whisker pathway of the naturally behaving animal. For instance, within a typical whisk involving environmental contact on several whiskers, we would expect the upstream signals to be composed of a battery of temporally interrelated, relatively brief and fairly stereotypical bursts of activity, rather than the more protracted signal streams that have been shown to arise during unmodulated whisking (Szwed et al 2003; Arabzadeh et al. 2005; Yu et al. 2006). Our results therefore have implications for the design of appropriate stimuli for use in future studies of the whisker processing pathways as well as for divining the functions of the vibrissal somatosensory system. We are currently developing simulation and robotic models of the neural processing of whisking signals (Mitchinson et al. 2004; Pearson et al. 2005) and of whisking control (Mitchinson et al. 2006) designed to test the efficacy of the rat's active whisking control strategies in relation to information gain and to evaluate their probable effects on upstream neural systems.
4. Material and methods
Adult male dystrophic Royal College of Surgeons rats were used in all experiments. These animals display not only normal whisker function, but also a genetically induced retinal degeneration (dystrophy) such that they had minimal visual capacity at the time of testing (Hetherington et al. 2000). Three animals were bilaterally implanted with subcutaneous EMG electrodes in the intrinsic muscle of each mystacial pad following a method similar to that described by Berg & Kleinfeld (2003b). These electrodes ran to a connector fixed to the top of the head, where a small telemetry transmitter could be attached during testing. For the high-speed video experiments, multiple, 4 s recordings of rats exploring a purpose-built arena were taken opportunistically using a Photron Fastcam PCI at 250 frames per second (fps). Movements of selected vibrissae were manually tracked at full resolution by multiple judges using a custom-built software tool (see video 1 of electronic supplementary material), and these data were used to compute estimates of protraction onset and cessation (figure 2), or of the full whisking cycle (figure 3). Time of initial contact with an obstacle was identified by inspection of relevant frames. For the paired normal-speed video/EMG recordings, animals were placed in a rectangular arena (400×380×80 mm) with a glass lid and floor. A domestic DV camera (25 fps) mounted above the arena allowed automated tracking of the location, bearing and pitch of the rat's head relative to the positions of the arena walls (see video 3 of electronic supplementary material). Recorded EMG signals were digitized and processed to extract activity in the appropriate frequency band for whisking. Two signals were obtained: one fast-varying (filtered in the range 2–20 Hz) and used to verify the correlation with whisker protraction in high-speed video (figure 3 and video 2 of electronic supplementary material), and the other slow-varying (low-pass filtered at 2 Hz to remove phase information) and used as the whisking amplitude measure for all further statistical analyses. Review of paired video/EMG recordings allowed the elimination of periods of experimental noise, ambiguous tracking, irrelevant behaviour (e.g. grooming), extreme head pitch and weak or absent whisking. Two in every three video frames were then discarded to give the desired sample rate of 8.33 Hz. Since the whisking amplitude measures were strongly autocorrelated within sessions, statistical tests used repeated measures ANOVA computed over per-session values with subset (NEAR/FAR) and session as within-subject factors. α was 0.05 and, for the ANOVA tests, was partial Bonferroni corrected to 0.048. Reported p-values are two-tailed. All procedures were carried out with local ethics committee and UK Home Office approval under the terms of the Animals (Scientific Procedures) Act 1986. A detailed description of these methods is presented in the electronic supplementary material.
Acknowledgments
The authors wish to thank the other members of the ‘Whiskerbot’ project (http://www.whiskerbot.org)—C. Melhuish, K. Gurney, P. Redgrave, A. G. Pipe, M. Pearson and I. Gilhespy—for discussions that led to the inception of this study. B.M. was supported by an EPSRC project grant and the EU FP6 ‘ICEA’ project, C.J.M. by an MRC research grant, and R.A.G. by an EPSRC Doctoral Training Award. Technical assistance was provided by M. Simkins, A. Ham, M. Port, M. Benn and L. Hetherington.
Supplementary Material
Additional details of the methods and of the results obtained with individual animals. The order of presentation is (i) detailed methods, (ii) additional results, and (iii) commentary on the video material
An example of bilateral tracking of whisker movement during a sequence in which a rat makes contact with a vertical surface, on a forward whisker, whilst proceeding across a featureless floor. Replay is at 1/50th normal speed. Frames from this sequence were also shown in figure 1. See supplementary information for further details
One of the high-speed clips used to verify the relationship between recorded EMG and whisker angle, presented here with additional information overlaid. Replay is at 1/10th normal speed. The histograms and stereo soundtrack both indicate strength of whisker protraction as recorded by implanted electrodes. See supplementary information for further details
A short clip of the normal-speed video to illustrate the automated tracking of the two tags on the animal head along with tracking of the nose. The stereo soundtrack contains the synchronised raw EMG signals
A short clip of high-speed video, with both side-on and overhead views, to illustrate the strong asymmetry we observe when the animal approaches a wall at an acute angle. Replay is at 1/10th normal speed. A frame from this sequence was shown in figure 5. See supplementary information for further details
References
- Ahissar E, Arieli A. Figuring space by time. Neuron. 2001;32:185–201. doi: 10.1016/s0896-6273(01)00466-4. doi:10.1016/S0896-6273(01)00466-4 [DOI] [PubMed] [Google Scholar]
- Aloimonos J.Y, Weiss I, Bandopadhay A. Active vision. Int. J. Comput. Vis. 1988;1:333–356. doi:10.1007/BF00133571 [Google Scholar]
- Arabzadeh E, Zorzin E, Diamond M.E. Neuronal encoding of texture in the whisker sensory pathway. PLoS Biol. 2005;3:e17. doi: 10.1371/journal.pbio.0030017. doi:10.1371/journal.pbio.0030017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard D.H. Animate vision. Artif. Intell. 1991;48:57–96. doi:10.1016/0004-3702(91)90080-4 [Google Scholar]
- Berg R.W, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J. Neurophysiol. 2003;89:104–117. doi: 10.1152/jn.00600.2002. doi:10.1152/jn.00600.2002 [DOI] [PubMed] [Google Scholar]
- Berg R.W, Kleinfeld D. Vibrissa movement elicited by rhythmic electrical microstimulation to motor cortex in the aroused rat mimics exploratory whisking. J. Neurophysiol. 2003b;90:2950–2963. doi: 10.1152/jn.00511.2003. doi:10.1152/jn.00511.2003 [DOI] [PubMed] [Google Scholar]
- Bermejo R, Vyas A, Zeigler H.P. Topography of rodent whisking-I. Two-dimensional monitoring of whisker movements. Somatosens. Mot. Res. 2002;19:341–346. doi: 10.1080/0899022021000037809. doi:10.1080/0899022021000037809 [DOI] [PubMed] [Google Scholar]
- Brecht M, Preilowski B, Merzenich M.M. Functional architecture of the mystacial vibrissae. Behav. Brain Res. 1997;84:81–97. doi: 10.1016/s0166-4328(97)83328-1. doi:10.1016/S0166-4328(97)83328-1 [DOI] [PubMed] [Google Scholar]
- Bruno R.M, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312:1622–1627. doi: 10.1126/science.1124593. doi:10.1126/science.1124593 [DOI] [PubMed] [Google Scholar]
- Carvell G.E, Simons D.J. Biometric analyses of vibrissal tactile discrimination in the rat. J. Neurosci. 1990;10:2638–2648. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvell G.E, Simons D.J, Lichtenstein S.H, Bryant P. Electromyographic activity of mystacial pad musculature during whisking behavior in the rat. Somatosens. Mot. Res. 1991;8:159–164. doi: 10.3109/08990229109144740. [DOI] [PubMed] [Google Scholar]
- Chapman C.E. Active versus passive touch: factors influencing the transmission of somatosensory signals to primary somatosensory cortex. Can. J. Physiol. Pharmacol. 1994;72:558–570. doi: 10.1139/y94-080. [DOI] [PubMed] [Google Scholar]
- Cramer N.P, Keller A. Cortical control of a whisking central pattern generator. J. Neurophysiol. 2006;96:209–217. doi: 10.1152/jn.00071.2006. doi:10.1152/jn.00071.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvergne C, Zerari-Mailly F, Buisseret P, Buisseret-Delmas C, Pinganaud G. The sensory trigeminal complex projects contralaterally to the facial motor and the accessory abducens nuclei in the rat. Neurosci. Lett. 2002;329:169–172. doi: 10.1016/s0304-3940(02)00656-0. doi:10.1016/S0304-3940(02)00656-0 [DOI] [PubMed] [Google Scholar]
- Derdikman D, Szwed M, Bagdasarian K, Knutsen P.M, Pietr M, Yu C, Arieli A, Ahissar E. Active construction of percepts about object location. Novartis Found. Symp. 2006;270:4–14. [PubMed] [Google Scholar]
- Dyck R.H. In: The behavior of the laboratory rat: a handbook with tests. Wishaw I.Q, Kolb B, editors. Oxford University Press; Oxford, UK: 2005. pp. 81–89. Vibrissae. [Google Scholar]
- Feldman D.E, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. doi:10.1126/science.1115807 [DOI] [PubMed] [Google Scholar]
- Ferezou I, Bolea S, Petersen C.C. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron. 2006;50:617–629. doi: 10.1016/j.neuron.2006.03.043. doi:10.1016/j.neuron.2006.03.043 [DOI] [PubMed] [Google Scholar]
- Gao P, Bermejo R, Zeigler H.P. Whisker deafferentation and rodent whisking patterns: behavioral evidence for a central pattern generator. J. Neurosci. 2001;21:5374–5380. doi: 10.1523/JNEUROSCI.21-14-05374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J.J. Observations on active touch. Psychol. Rev. 1962;69:477–491. doi: 10.1037/h0046962. doi:10.1037/h0046962 [DOI] [PubMed] [Google Scholar]
- Gibson J.M, Welker W.I. Quantitative studies of stimulus coding in first-order vibrissa afferents of rats 1. Receptive field properties and threshold distributions. Somatosens. Res. 1983a;1:51–67. doi: 10.3109/07367228309144540. [DOI] [PubMed] [Google Scholar]
- Gibson J.M, Welker W.I. Quantitative studies of stimulus coding in first-order vibrissa afferents of rats 2. Adaptation and coding of stimulus parameters. Somatosens. Res. 1983b;1:95–117. doi: 10.3109/07367228309144543. [DOI] [PubMed] [Google Scholar]
- Gustafson J.W, Felbain-Keramidas S.L. Behavioral and neural approaches to the function of the mystacial vibrissae. Psychol. Bull. 1977;84:477–488. doi:10.1037/0033-2909.84.3.477 [PubMed] [Google Scholar]
- Harris J.A, Petersen R.S, Diamond M.E. Distribution of tactile learning and its neural basis. Proc. Natl Acad. Sci. USA. 1999;96:7587–7591. doi: 10.1073/pnas.96.13.7587. doi:10.1073/pnas.96.13.7587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M.J. Active sensing capabilities of the rat whisker system. Auton. Robots. 2001;11:249–254. doi:10.1023/A:1012439023425 [Google Scholar]
- Hartmann M.J, Assad C, Rasnow B, Bower J.M. Applications of video mixing and digital overlay to neuroethology. J. Neurosci. Methods. 2000;21:385–391. doi: 10.1006/meth.2000.1025. [DOI] [PubMed] [Google Scholar]
- Hetherington L, Benn M, Coffey P.J, Lund R.D. Sensory capacity of the royal college of surgeons rat. Invest Ophthalmol. Vis. Sci. 2000;41:3979–3983. [PubMed] [Google Scholar]
- Jones L.M, Depireux D.A, Simons D.J, Keller A. Robust temporal coding in the trigeminal system. Science. 2004;304:1986–1989. doi: 10.1126/science.1097779. doi:10.1126/science.1097779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond M.E. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr. Opin. Neurobiol. 2006;16:435–444. doi: 10.1016/j.conb.2006.06.009. doi:10.1016/j.conb.2006.06.009 [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Berg R.W, O'Connor S.M. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosens. Mot. Res. 1999;16:69–88. doi: 10.1080/08990229970528. doi:10.1080/08990229970528 [DOI] [PubMed] [Google Scholar]
- Knutsen P.M, Derdikman D, Ahissar E. Tracking whisker and head movements in unrestrained behaving rodents. J. Neurophys. 2004;93:2294–2301. doi: 10.1152/jn.00718.2004. doi:10.1152/jn.00718.2004 [DOI] [PubMed] [Google Scholar]
- Li Y.Q, Takada M, Kaneko T, Mizuno N. Distribution of GABAergic and glycinergic premotor neurons projecting to the facial and hypoglossal nuclei in the rat. J. Comp. Neurol. 1997;378:283–294. doi: 10.1002/(sici)1096-9861(19970210)378:2<283::aid-cne10>3.0.co;2-t. doi:10.1002/(SICI)1096-9861(19970210)378:2<283::AID-CNE10>3.0.CO;2-T [DOI] [PubMed] [Google Scholar]
- Lichtenstein S.H, Carvell G.E, Simons D.J. Responses of rat trigeminal ganglion neurons to movements of vibrissae in different directions. Somatosens. Mot. Res. 1990;7:47–65. doi: 10.3109/08990229009144697. [DOI] [PubMed] [Google Scholar]
- Lungarella M, Pegors T, Bulwinkle D, Sporns O. Methods for quantifying the informational structure of sensory and motor data. Neuroinformatics. 2005;3:243–262. doi: 10.1385/NI:3:3:243. doi:10.1385/NI:3:3:243 [DOI] [PubMed] [Google Scholar]
- McHaffie J.G, Stanford T.R, Stein B.E, Coizet V, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28:401–407. doi: 10.1016/j.tins.2005.06.006. doi:10.1016/j.tins.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Mitchinson B, Gurney K.N, Redgrave P, Melhuish C, Pipe A.G, Pearson M, Gilhespy I, Prescott T.J. Empirically inspired simulated electro-mechanical model of the rat mystacial follicle–sinus complex. Proc. R. Soc. B. 2004;271:2509–2516. doi: 10.1098/rspb.2004.2882. doi:10.1098/rspb.2004.2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchinson, B., Pearson, M., Melhuish, C. & Prescott, T. J. 2006 A model of sensorimotor coordination in the rat whisker system. In From animals to animats 9: Proc. 9th Int. Conf. on Simulation of Adaptive Behaviour, LNAI, vol. 4095 (eds S. Nolfi et al). Berlin, Germany: Springer.
- Nguyen Q.T, Kleinfeld D. Positive feedback in a brainstem tactile sensorimotor loop. Neuron. 2005;45:447–457. doi: 10.1016/j.neuron.2004.12.042. doi:10.1016/j.neuron.2004.12.042 [DOI] [PubMed] [Google Scholar]
- Pearson M, Gilhespy I, Melhuish C, Mitchinson B, Nabouche M, Pipe A.G, Prescott T.J. A biomimetic haptic sensor. Int. J. Adv. Robot. Syst. 2005;2:335–343. [Google Scholar]
- Prescott T.J, Mitchinson B, Redgrave P, Melhuish C, Dean P. Society for Neuroscience; Washington, DC: 2005. Three-dimensional reconstruction of whisking patterns in freely moving rats. Abstract number 625.3. [Google Scholar]
- Prescott T.J, Redgrave P, Gurney K.N. Layered control architectures in robots and vertebrates. Adapt. Behav. 1999;7:99–127. [Google Scholar]
- Sachdev R.N, Berg R.W, Champney G, Kleinfeld D, Ebner F.F. Unilateral vibrissa contact: changes in amplitude but not timing of rhythmic whisking. Somatosens. Mot. Res. 2003;20:163–169. doi: 10.1080/08990220311000405208. doi:10.1080/08990220311000405208 [DOI] [PubMed] [Google Scholar]
- Shoykhet M, Doherty D, Simons D.J. Coding of deflection velocity and amplitude by whisker primary afferent neurons: implications for higher level processing. Somatosens. Mot. Res. 2000;17:171–180. doi: 10.1080/08990220050020580. doi:10.1080/08990220050020580 [DOI] [PubMed] [Google Scholar]
- Smith A.M, Gosselin G, Houde B. Deployment of fingertip forces in tactile exploration. Exp. Brain Res. 2002;147:209–218. doi: 10.1007/s00221-002-1240-4. doi:10.1007/s00221-002-1240-4 [DOI] [PubMed] [Google Scholar]
- Szwed M, Bagdasarian K, Ahissar E. Encoding of vibrissal active touch. Neuron. 2003;40:621–630. doi: 10.1016/s0896-6273(03)00671-8. doi:10.1016/S0896-6273(03)00671-8 [DOI] [PubMed] [Google Scholar]
- Towal R.B, Hartmann M.J. Right–left asymmetries in whisking behavior of rats anticipate head movements. J. Neurosci. 2006;26:8838–8846. doi: 10.1523/JNEUROSCI.0581-06.2006. doi:10.1523/JNEUROSCI.0581-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S.B. The function of the vibrissae in the behaviour of the white rat. Behav. Monogr. 1912;1:1–82. [Google Scholar]
- Yu C, Derdikman D, Haidarliu S, Ahissar E. Parallel thalamic pathways for whisking and touch signals in the rat. PLoS Biol. 2006;4:e124. doi: 10.1371/journal.pbio.0040124. doi:10.1371/journal.pbio.0040124 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional details of the methods and of the results obtained with individual animals. The order of presentation is (i) detailed methods, (ii) additional results, and (iii) commentary on the video material
An example of bilateral tracking of whisker movement during a sequence in which a rat makes contact with a vertical surface, on a forward whisker, whilst proceeding across a featureless floor. Replay is at 1/50th normal speed. Frames from this sequence were also shown in figure 1. See supplementary information for further details
One of the high-speed clips used to verify the relationship between recorded EMG and whisker angle, presented here with additional information overlaid. Replay is at 1/10th normal speed. The histograms and stereo soundtrack both indicate strength of whisker protraction as recorded by implanted electrodes. See supplementary information for further details
A short clip of the normal-speed video to illustrate the automated tracking of the two tags on the animal head along with tracking of the nose. The stereo soundtrack contains the synchronised raw EMG signals
A short clip of high-speed video, with both side-on and overhead views, to illustrate the strong asymmetry we observe when the animal approaches a wall at an acute angle. Replay is at 1/10th normal speed. A frame from this sequence was shown in figure 5. See supplementary information for further details