Abstract
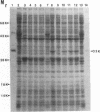
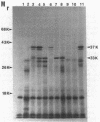
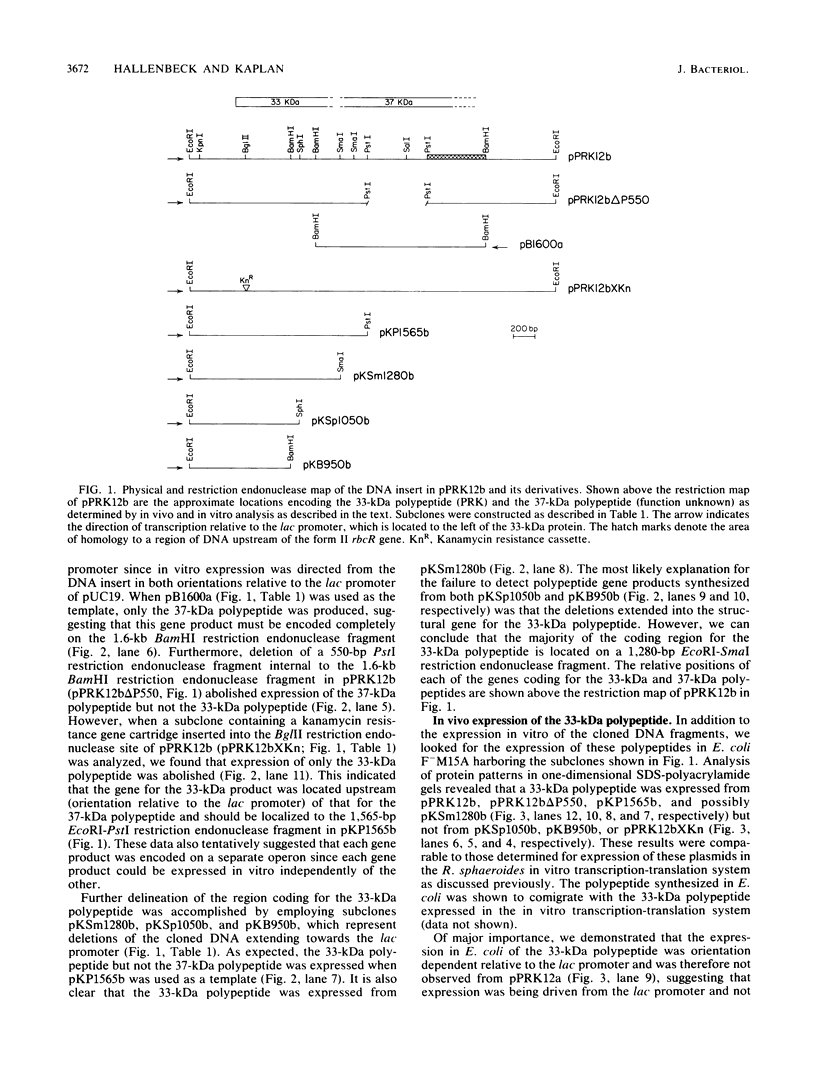
A 3.4-kilobase EcoRI restriction endonuclease fragment has been cloned from the facultatively photoheterotrophic bacterium Rhodobacter sphaeroides and shown to contain the structural gene (prkA) for phosphoribulokinase (PRK) activity. The PRK activity was characterized in Escherichia coli, and the product of the reaction was identified. The prkA gene was localized to a 1,565-base-pair EcoRI-PstI restriction endonuclease fragment and gave rise to a 33-kilodalton polypeptide both in vivo and in vitro. The gene product produced in E. coli was shown to be identical to the gene product produced in R. sphaeroides. The amino acid sequence for the amino-terminal region deduced from the DNA sequence confirmed that derived for partially purified PRK derived from both E. coli and R. sphaeroides. In addition, the 3.4-kilobase EcoRI restriction endonuclease fragment coded for a 37-kilodalton polypeptide of unknown function, and preliminary evidence indicates that this DNA fragment is linked to genes coding for other activities significant in photosynthetic carbon assimilation. The genetic organization and proposed operon structure of this DNA fragment are discussed.
Full text
PDF


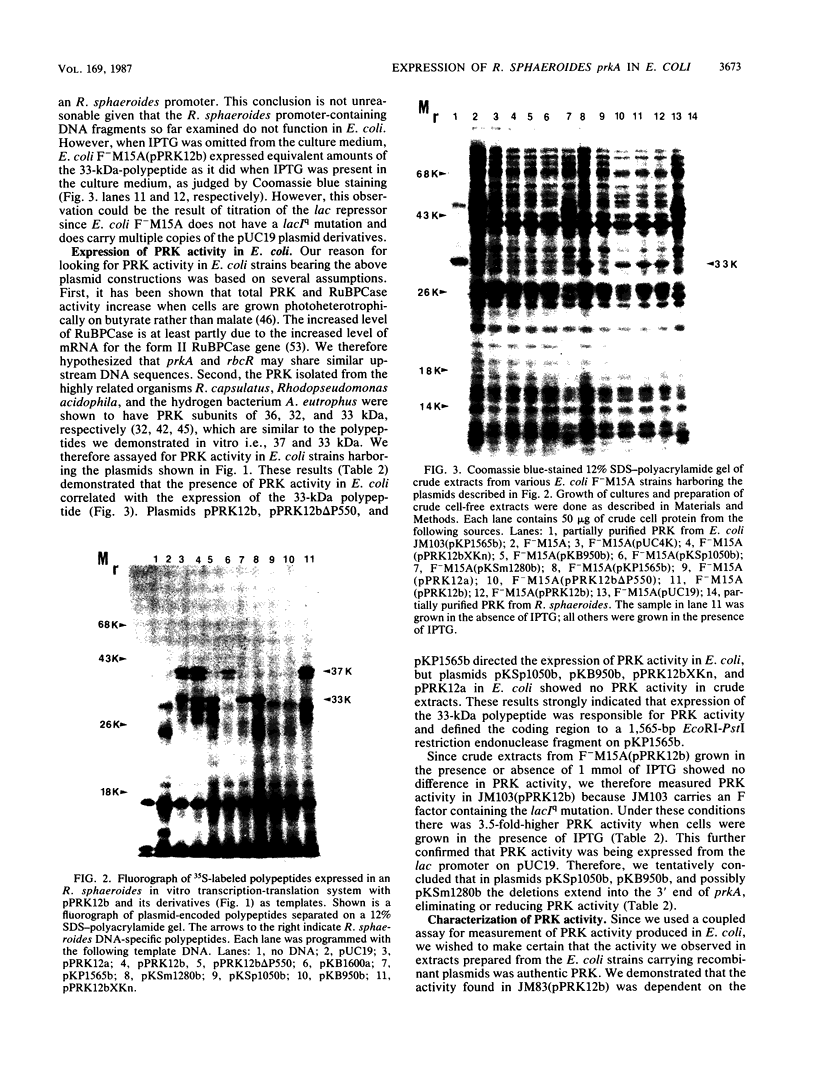
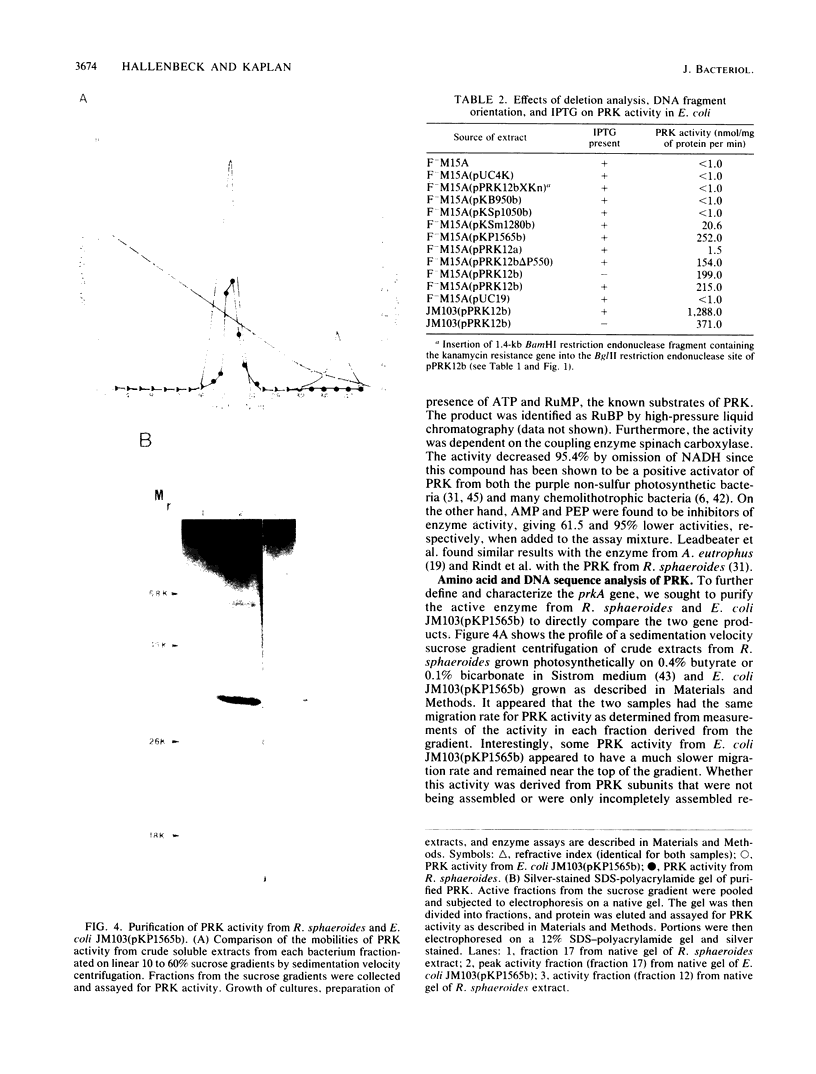




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelal A. T., Schlegel H. G. Purification and regulatory properties of phosphoribulokinase from Hydrogenomonas eutropha H 16. Biochem J. 1974 Jun;139(3):481–489. doi: 10.1042/bj1390481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard R. W., MacElroy R. D. Phosphoenolpyruvate, a new inhibitor of phosphoribulokinase in pseudomonas facilis. Biochem Biophys Res Commun. 1971 Aug 6;44(3):614–618. doi: 10.1016/s0006-291x(71)80127-4. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chory J., Kaplan S. The in vitro transcription-translation of DNA and RNA templates by extracts of Rhodopseudomonas sphaeroides. Optimization and comparison of template specificity with Escherichia coli extracts and in vivo synthesis. J Biol Chem. 1982 Dec 25;257(24):15110–15121. [PubMed] [Google Scholar]
- Crawford N. A., Sutton C. W., Yee B. C., Johnson T. C., Carlson D. C., Buchanan B. B. Contrasting modes of photosynthetic enzyme regulation in oxygenic and anoxygenic prokaryotes. Arch Microbiol. 1984 Oct;139(2-3):124–129. doi: 10.1007/BF00401986. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Different molecular forms of D-ribulose-1,5-bisphosphate carboxylase from Rhodopseudomonas sphaeroides. J Biol Chem. 1977 Feb 10;252(3):943–949. [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Isolation and preliminary characterization of two forms of ribulose 1,5-bisphosphate carboxylase from Rhodopseudomonas capsulata. J Bacteriol. 1977 Dec;132(3):818–823. doi: 10.1128/jb.132.3.818-823.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. L., Tabita F. R. Isolation of the Rhodopseudomonas sphaeroides form I ribulose 1,5-bisphosphate carboxylase/oxygenase large and small subunit genes and expression of the active hexadecameric enzyme in Escherichia coli. Gene. 1986;44(2-3):271–278. doi: 10.1016/0378-1119(86)90191-5. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Im D. S., Friedrich C. G. Fluoride, hydrogen, and formate activate ribulosebisphosphate carboxylase formation in Alcaligenes eutrophus. J Bacteriol. 1983 May;154(2):803–808. doi: 10.1128/jb.154.2.803-808.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanneau Y., Tabita F. R. Independent regulation of synthesis of form I and form II ribulose bisphosphate carboxylase-oxygenase in Rhodopseudomonas sphaeroides. J Bacteriol. 1986 Feb;165(2):620–624. doi: 10.1128/jb.165.2.620-624.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintworth R., Husemann M., Salnikow J., Bowien B. Chromosomal and plasmid locations for phosphoribulokinase genes in Alcaligenes eutrophus. J Bacteriol. 1985 Nov;164(2):954–956. doi: 10.1128/jb.164.2.954-956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leadbeater L., Bowien B. Control of autotrophic carbon assimilation in Alcaligenes eutrophus by inactivation and reactivation of phosphoribulokinase. J Bacteriol. 1984 Jan;157(1):95–99. doi: 10.1128/jb.157.1.95-99.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M. T., Gest H. Growth of the photosynthetic bacterium Rhodopseudomonas capsulata chemoautotrophically in darkness with H2 as the energy source. J Bacteriol. 1979 Jan;137(1):524–530. doi: 10.1128/jb.137.1.524-530.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat L. E., Reznikoff W. S. In vitro analysis of the Escherichia coli RNA polymerase interaction with wild-type and mutant lactose promoters. J Mol Biol. 1978 Nov 15;125(4):467–490. doi: 10.1016/0022-2836(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Muller E. D., Chory J., Kaplan S. Cloning and characterization of the gene product of the form II ribulose-1,5-bisphosphate carboxylase gene of Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jan;161(1):469–472. doi: 10.1128/jb.161.1.469-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M. A., Milanez S., Stringer C. D., Hartman F. C. Purification and characterization of ribulose-5-phosphate kinase from spinach. Arch Biochem Biophys. 1986 Feb 15;245(1):14–23. doi: 10.1016/0003-9861(86)90185-2. [DOI] [PubMed] [Google Scholar]
- Quivey R. G., Jr, Tabita F. R. Cloning and expression in Escherichia coli of the form II ribulose 1,5-bisphosphate carboxylase/oxygenase gene from Rhodopseudomonas sphaeroides. Gene. 1984 Nov;31(1-3):91–101. doi: 10.1016/0378-1119(84)90198-7. [DOI] [PubMed] [Google Scholar]
- Rindt K. P., Ohmann E. NADH and AMP as allosteric effectors of ribulose-5-phosphate kinase in Rhodopseudomonas spheroides. Biochem Biophys Res Commun. 1969 Aug 7;36(3):357–364. doi: 10.1016/0006-291x(69)90572-5. [DOI] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Otto K., Müller-Hill B. pUR222, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4087–4098. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- SISTROM W. R. The kinetics of the synthesis of photopigments in Rhodopseudomonas spheroides. J Gen Microbiol. 1962 Sep;28:607–616. doi: 10.1099/00221287-28-4-607. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Sugiura M. The gene for the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is located close to the gene for the large subunit in the cyanobacterium Anacystis nidulans 6301. Nucleic Acids Res. 1983 Oct 25;11(20):6957–6964. doi: 10.1093/nar/11.20.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Yamada C., Takahata N., Sugiura M. Molecular cloning and sequence analysis of the cyanobacterial gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4050–4054. doi: 10.1073/pnas.80.13.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M., Davidson E., Marrs B. L. Depression of the synthesis of the intermediate and large forms of ribulose-1,5-bisphosphate carboxylase/oxygenase in Rhodopseudomonas capsulata. Arch Microbiol. 1984 Jul;138(3):233–236. doi: 10.1007/BF00402127. [DOI] [PubMed] [Google Scholar]
- Siebert K., Schobert P., Bowien B. Purification, some catalytic and molecular properties of phosphoribulokinase from Alcaligenes eutrophus. Biochim Biophys Acta. 1981 Mar 13;658(1):35–44. doi: 10.1016/0005-2744(81)90247-3. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., Caruso P., Whitman W. Facile assay of enzymes unique to the Calvin cycle in intact cells, with special reference to ribulose 1,5-bisphosphate carboxylase. Anal Biochem. 1978 Feb;84(2):462–472. doi: 10.1016/0003-2697(78)90064-7. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. I. Levels, purification, and effects of metallic ions. J Biol Chem. 1974 Jun 10;249(11):3453–3458. [PubMed] [Google Scholar]
- Tabita F. R. Pyridine nucleotide control and subunit structure of phosphoribulokinase from photosynthetic bacteria. J Bacteriol. 1980 Sep;143(3):1275–1280. doi: 10.1128/jb.143.3.1275-1280.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weaver K. E., Tabita F. R. Isolation and partial characterization of Rhodopseudomonas sphaeroides mutants defective in the regulation of ribulose bisphosphate carboxylase/oxygenase. J Bacteriol. 1983 Nov;156(2):507–515. doi: 10.1128/jb.156.2.507-515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zhu Y. S., Kaplan S. Effects of light, oxygen, and substrates on steady-state levels of mRNA coding for ribulose-1,5-bisphosphate carboxylase and light-harvesting and reaction center polypeptides in Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jun;162(3):925–932. doi: 10.1128/jb.162.3.925-932.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]