Abstract
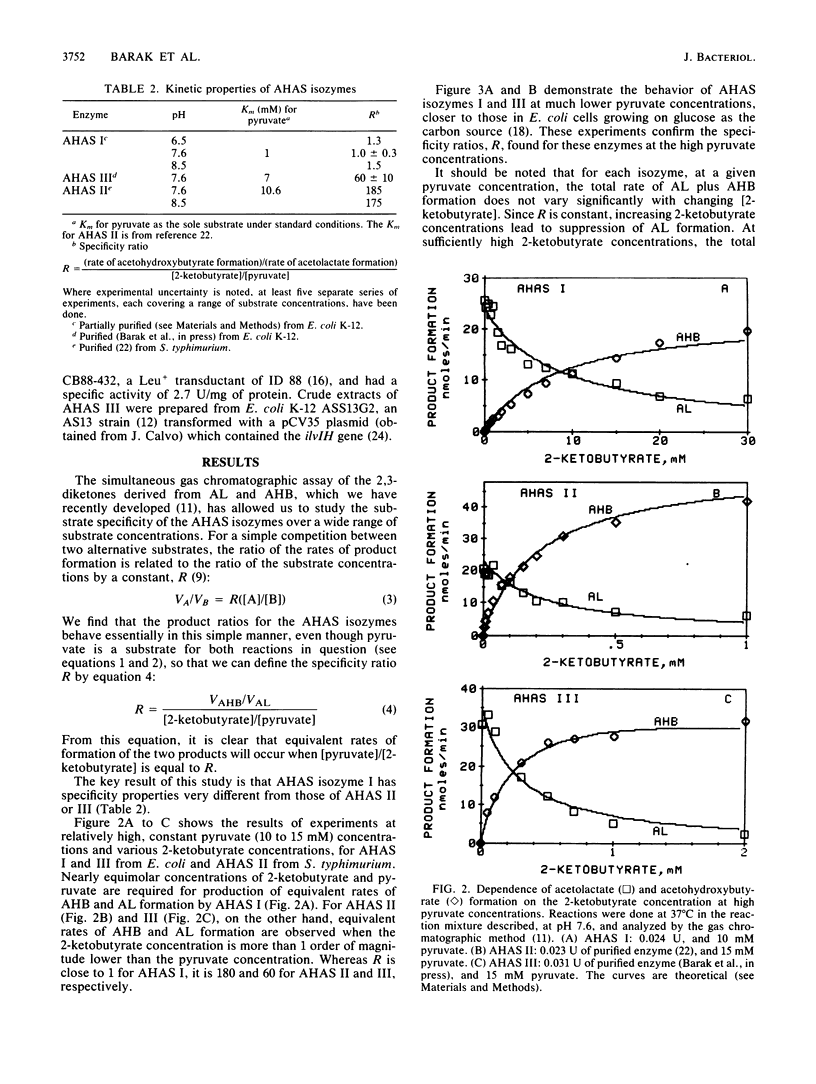
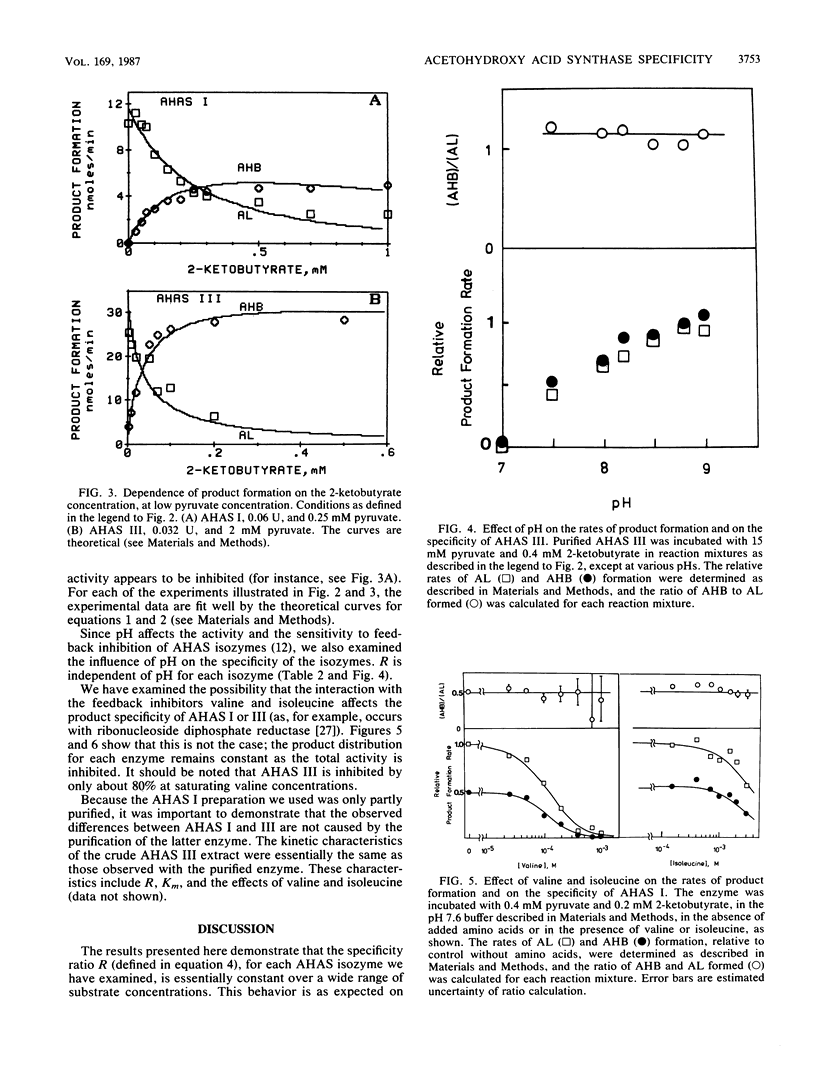
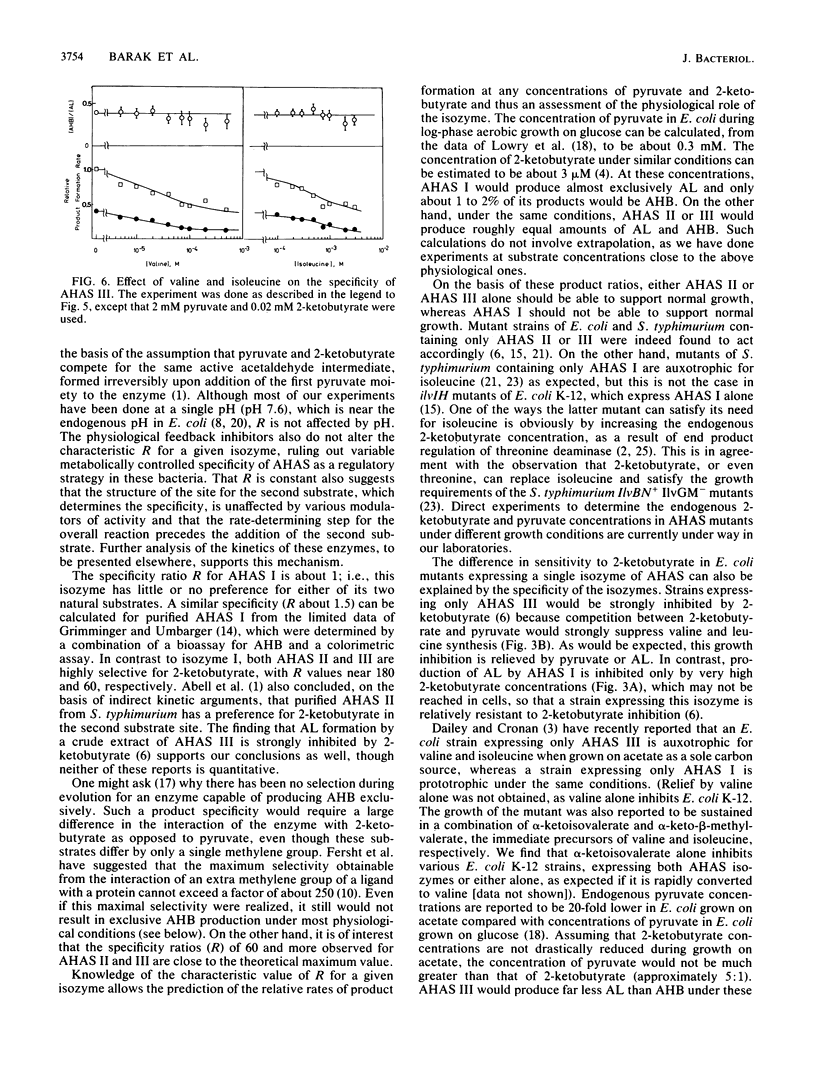
The rates of formation of the two alternative products of acetohydroxy acid synthase (AHAS) have been determined by a new analytical method (N. Gollop, Z. Barak, and D. M. Chipman, Anal. Biochem., 160:323-331, 1987). For each of the three distinct isozymes of AHAS in Escherichia coli and Salmonella typhimurium, a specificity ratio, R, was defined: Formula: see text, which is constant over a wide range of substrate concentrations. This is consistent with competition between pyruvate and 2-ketobutyrate for an active acetaldehyde intermediate formed irreversibly after addition of the first pyruvate moiety to the enzyme. Isozyme I showed no product preference (R = 1), whereas isozymes II and III form acetohydroxybutyrate (AHB) at approximately 180- and 60-fold faster rates, respectively, than acetolactate (AL) at equal pyruvate and 2-ketobutyrate concentrations. R values higher than 60 represent remarkably high specificity in favor of the substrate with one extra methylene group. In exponentially growing E. coli cells (under aerobic growth on glucose), which contain about 300 microM pyruvate and only 3 microM 2-ketobutyrate, AHAS I would produce almost entirely AL and only 1 to 2% AHB. However, isozymes II and III would synthesize AHB (on the pathway to Ile) and AL (on the pathway to valine-leucine) in essentially the ratio required for protein synthesis. The specificity ratio R of any AHAS isozyme was affected neither by the natural feedback inhibitors (Val, Ile) nor by the pH. On the basis of the specificities of the isozymes, the known regulation of AHAS I expression by the catabolite repression system, and the reported behavior of bacterial mutants containing single AHAS isozymes, we suggest that AHAS I enables a bacterium to cope with poor carbon sources, which lead to low endogenous pyruvate concentrations. Although AHAS II and III are well suited to producing the branched-chain amino acid precursors during growth on glucose, they would fail to provide appropriate quantities of AL when the concentration of pyruvate is relatively low.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dailey F. E., Cronan J. E., Jr Acetohydroxy acid synthase I, a required enzyme for isoleucine and valine biosynthesis in Escherichia coli K-12 during growth on acetate as the sole carbon source. J Bacteriol. 1986 Feb;165(2):453–460. doi: 10.1128/jb.165.2.453-460.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J., Dondon L., Danchin A. 2-Ketobutyrate: a putative alarmone of Escherichia coli. Mol Gen Genet. 1983;190(3):452–458. doi: 10.1007/BF00331076. [DOI] [PubMed] [Google Scholar]
- Eoyang L., Silverman P. M. Purification and subunit composition of acetohydroxyacid synthase I from Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):184–189. doi: 10.1128/jb.157.1.184-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H., Porter J. S., Slayman C. L., Kaback H. R. Quantitative measurements of membrane potential in Escherichia coli. Biochemistry. 1980 Jul 22;19(15):3585–3590. doi: 10.1021/bi00556a026. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Shindler J. S., Tsui W. C. Probing the limits of protein-amino acid side chain recognition with the aminoacyl-tRNA synthetases. Discrimination against phenylalanine by tyrosyl-tRNA synthetases. Biochemistry. 1980 Nov 25;19(24):5520–5524. doi: 10.1021/bi00565a009. [DOI] [PubMed] [Google Scholar]
- Gollop N., Barak Z., Chipman D. M. A method for simultaneous determination of the two possible products of acetohydroxy acid synthase. Anal Biochem. 1987 Feb 1;160(2):323–331. doi: 10.1016/0003-2697(87)90054-6. [DOI] [PubMed] [Google Scholar]
- Gollop N., Chipman D. M., Barak Z. Inhibition of acetohydroxy acid synthase by leucine. Biochim Biophys Acta. 1983 Oct 17;748(1):34–39. doi: 10.1016/0167-4838(83)90024-9. [DOI] [PubMed] [Google Scholar]
- Gordeev V. K., Turkov M. I. Kompleksnaia reguliatsiia aktivnosti ilv-genov v kletkakh Escherichia coli K-12. Genetika. 1985 Jul;21(7):1077–1089. [PubMed] [Google Scholar]
- Grimminger H., Umbarger H. E. Acetohydroxy acid synthase I of Escherichia coli: purification and properties. J Bacteriol. 1979 Feb;137(2):846–853. doi: 10.1128/jb.137.2.846-853.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Lamberti A., Iaccarino M. The acetolactate synthase isoenzymes of Escherichia coli K-12. Mol Gen Genet. 1977 Nov 4;156(1):17–25. doi: 10.1007/BF00272247. [DOI] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago C. T., Sannia G., Marino G., Squires C. H., Calvo J. M., De Felice M. The ilvIH operon of Escherichia coli K-12. Identification of the gene products and recognition of the translational start by polypeptide microsequencing. Biochim Biophys Acta. 1985 Jan 29;824(1):74–79. doi: 10.1016/0167-4781(85)90031-4. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- Newman T., Friden P., Sutton A., Freundlich M. Cloning and expression of the ilvB gene of Escherichia coli K-12. Mol Gen Genet. 1982;186(3):378–384. doi: 10.1007/BF00729457. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Primerano D. A., Burns R. O. Metabolic basis for the isoleucine, pantothenate or methionine requirement of ilvG strains of Salmonella typhimurium. J Bacteriol. 1982 Jun;150(3):1202–1211. doi: 10.1128/jb.150.3.1202-1211.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. V., Van Dyk D. E., Vasta J. F., Kutny R. M. Purification and properties of Salmonella typhimurium acetolactate synthase isozyme II from Escherichia coli HB101/pDU9. Biochemistry. 1985 Aug 27;24(18):4952–4959. doi: 10.1021/bi00339a034. [DOI] [PubMed] [Google Scholar]
- Shaw K. J., Berg C. M., Sobol T. J. Salmonella typhimurium mutants defective in acetohydroxy acid synthases I and II. J Bacteriol. 1980 Mar;141(3):1258–1263. doi: 10.1128/jb.141.3.1258-1263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Wessler S. R., Calvo J. M. Physical characterization of the ilvHI operon of Escherichia coli K-12. J Bacteriol. 1981 Sep;147(3):797–804. doi: 10.1128/jb.147.3.797-804.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Freundlich M. Regulation of cyclic AMP of the ilvB-encoded biosynthetic acetohydroxy acid synthase in Escherichia coli K-12. Mol Gen Genet. 1980 Apr;178(1):179–183. doi: 10.1007/BF00267227. [DOI] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Burns R. O. Regulation of expression of the ilvB operon in Salmonella typhimurium. J Bacteriol. 1984 Dec;160(3):833–841. doi: 10.1128/jb.160.3.833-841.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felice M., Lago C. T., Squires C. H., Calvo J. M. Acetohydroxy acid synthase isoenzymes of Escherichia coli K12 and Salmonella typhimurium. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):251–256. [PubMed] [Google Scholar]



