Abstract
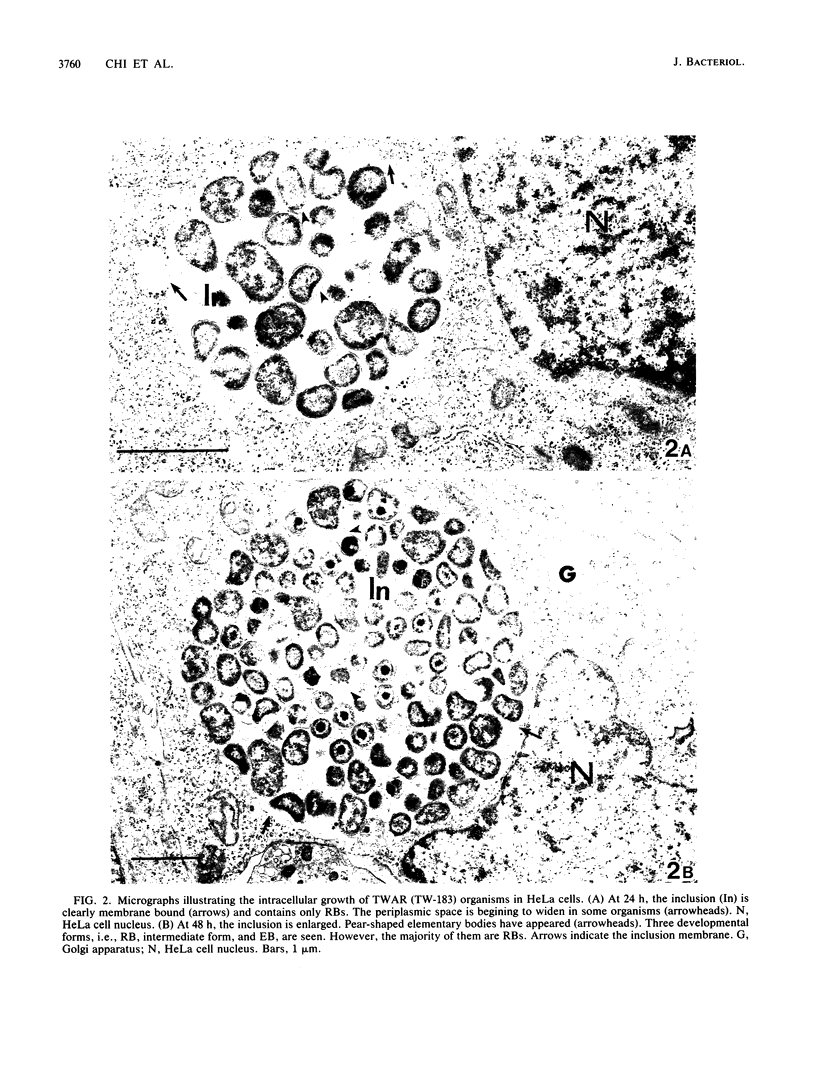
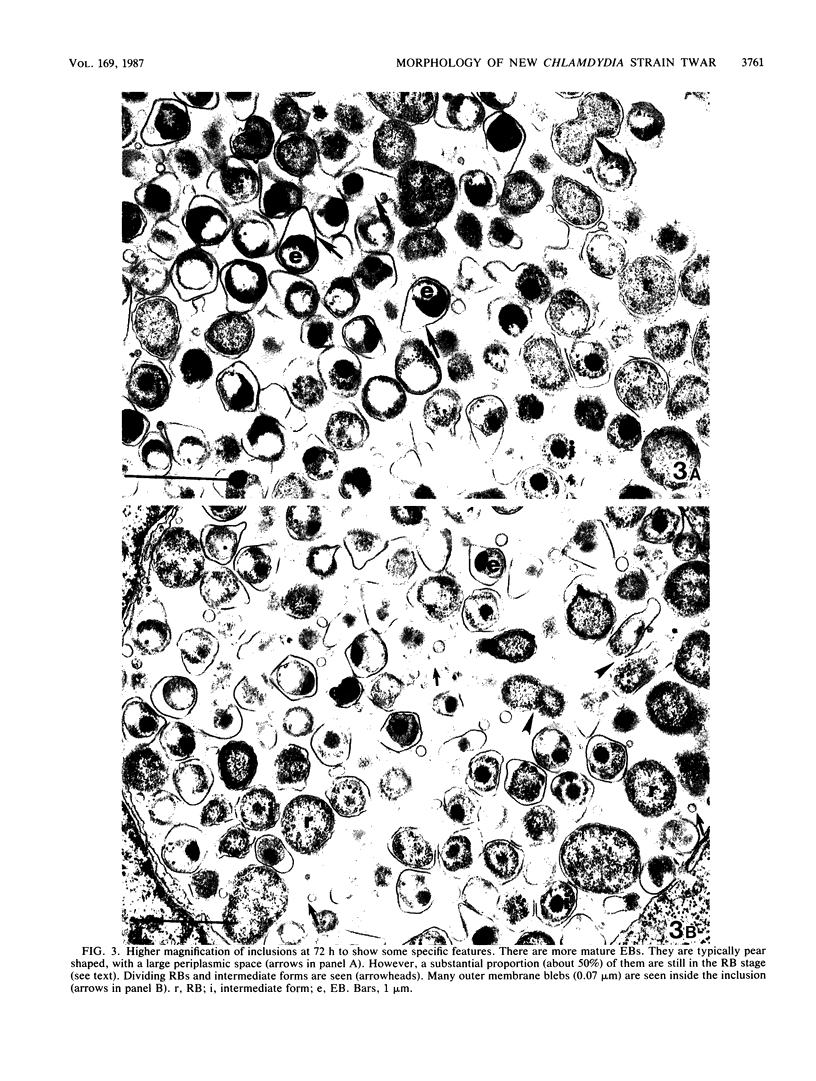
The ultrastructure of two prototype strains (TW-183 and AR-39) of Chlamydia sp. strain TWAR was described. The TWAR elementary body (EB) demonstrated a unique morphology and structure distinct from those of other chlamydial organisms. It was pleomorphic but typically pear shaped. The average size was 0.38 micron, with a long axis of 0.44 micron, a short axis of 0.31 micron, and a ratio of the long to the short axes of 1.42. The cytoplasmic mass was round, with an average diameter of 0.24 micron. There was a large periplasmic space. Small, round electron-dense bodies (0.05 micron in diameter), which were attached to the cytoplasm by a stringlike structure, were seen in the periplasmic space. These features are in contrast to those of other chlamydiae, which are typically round with a narrow or barely discernible periplasmic space. The TWAR reticulate body (RB) was morphologically and structurally similar to those of other Chlamydia species, having an average diameter of 0.51 micron and being circular in shape. The ultrastructural observations of the intracellular growth of TWAR in HeLa cells revealed that TWAR underwent the same developmental cycle as do other chlamydiae, i.e., transformation of EB to RB, multiplication by binary fission, and maturation by transformation of RB to EB via the intermediate-form stage.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron A. L., White H. J., Rank R. G., Soloff B. L. Target tissues associated with genital infection of female guinea pigs by the chlamydial agent of guinea pig inclusion conjunctivitis. J Infect Dis. 1979 Jan;139(1):60–68. doi: 10.1093/infdis/139.1.60. [DOI] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi E. Y., Lagunoff D., Koehler J. K. Freeze-fracture study of mast cell secretion. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2823–2827. doi: 10.1073/pnas.73.8.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Poffenroth L., Wilt J. C., Kordová N. Ultrastructural studies of Chlamydia psittaci 6BC in situ in yolk sac explants and L cells: a comparison with gram-negative bacteria. Can J Microbiol. 1975 Oct;21(10):1433–1447. doi: 10.1139/m75-215. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. OCCURENCE OF GLYCOGEN IN INCLUSIONS OF THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM-TRACHOMA AGENTS. J Infect Dis. 1965 Apr;115:186–196. doi: 10.1093/infdis/115.2.186. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Kuo C. C., Wang S. P., Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986 Jul 17;315(3):161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- Hargis A. M., Prieur D. J., Gaillard E. T. Chlamydial infection of the gastric mucosa in twelve cats. Vet Pathol. 1983 Mar;20(2):170–178. doi: 10.1177/030098588302000204. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y. Ultrastructural characterization and morphometric analysis of human eosinophil degranulation. J Cell Sci. 1985 Feb;73:33–48. doi: 10.1242/jcs.73.1.33. [DOI] [PubMed] [Google Scholar]
- Howard L., Orenstein N. S., King N. W. Purification on renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974 Jan;27(1):102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Chen H. H., Wang S. P., Grayston J. T. Identification of a new group of Chlamydia psittaci strains called TWAR. J Clin Microbiol. 1986 Dec;24(6):1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Chi E. Y. Ultrastructural study of Chlamydia trachomatis surface antigens by immunogold staining with monoclonal antibodies. Infect Immun. 1987 May;55(5):1324–1328. doi: 10.1128/iai.55.5.1324-1328.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. M., Swenson C. E., Schachter J. Ultrastructure of Chlamydia trachomatis infection of the mouse oviduct. J Ultrastruct Res. 1984 Sep;88(3):244–256. doi: 10.1016/s0022-5320(84)90122-9. [DOI] [PubMed] [Google Scholar]
- Popov V., Eb F., Lefebvre J. F., Orfila J., Viron A. Morphological and cytochemical study of Chlamydia with EDTA regressive technique and Gautier staining in ultrathin frozen sections of infected cell cultures: a comparison with embedded material. Ann Microbiol (Paris) 1978 Oct;129 B(3):313–337. [PubMed] [Google Scholar]
- Rowe L. W., Hedger R. S., Smale C. The isolation of a Chlamydia psittaci-like agent from a free-living African buffalo (Syncerus caffer). Vet Rec. 1978 Jul;103(1):13–14. doi: 10.1136/vr.103.1.13. [DOI] [PubMed] [Google Scholar]
- Stirling P., Richmond S. The developmental cycle of Chlamydia trachomatis in McCoy cells treated with cytochalasin B. J Gen Microbiol. 1977 May;100(1):31–42. doi: 10.1099/00221287-100-1-31. [DOI] [PubMed] [Google Scholar]
- Tamura A., Matsumoto A., Manire G. P., Higashi N. Electron microscopic observations on the structure of the envelopes of mature elementary bodies and developmental reticulate forms of Chlamydia psittaci. J Bacteriol. 1971 Jan;105(1):355–360. doi: 10.1128/jb.105.1.355-360.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanami Y., Yamada Y. Miniature cell formation in Chlamydia psittaci. J Bacteriol. 1973 Apr;114(1):408–412. doi: 10.1128/jb.114.1.408-412.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Gale J. L. Three new immunologic types of trachoma-inclusion conjunctivitis organisms. J Immunol. 1973 Mar;110(3):873–879. [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970 Sep;70(3):367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]