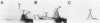Abstract
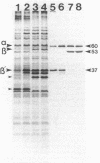
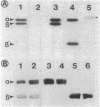
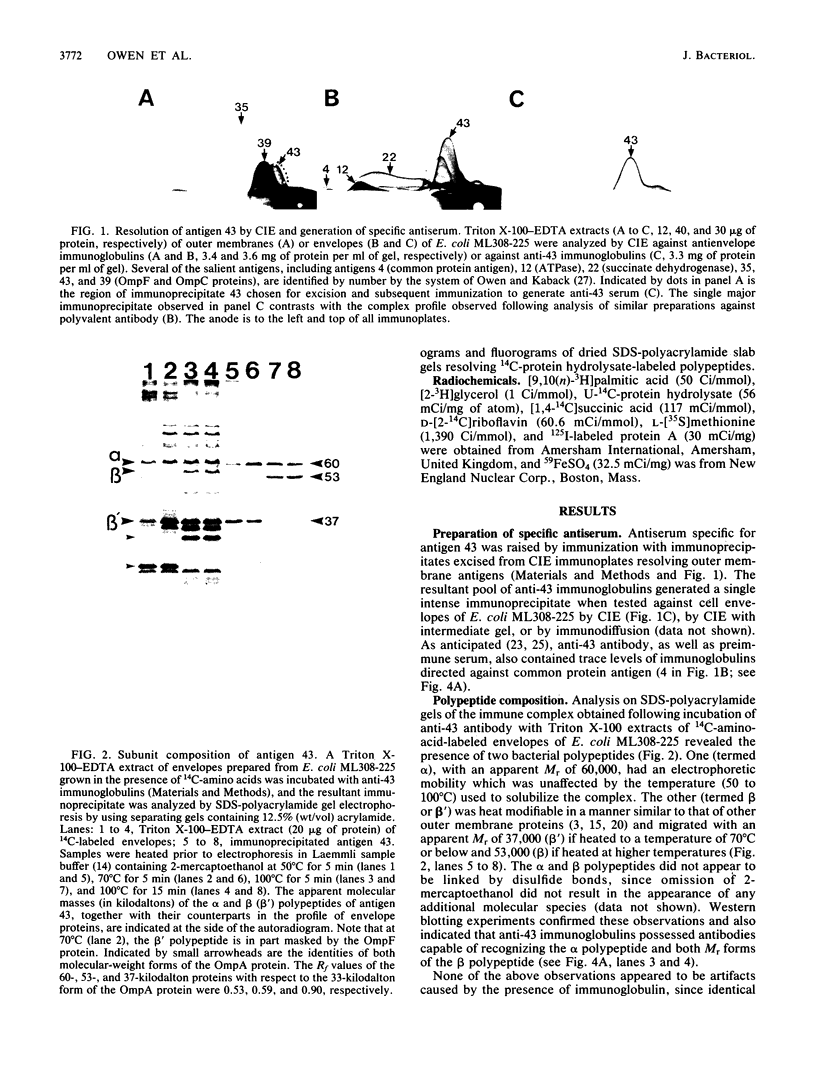
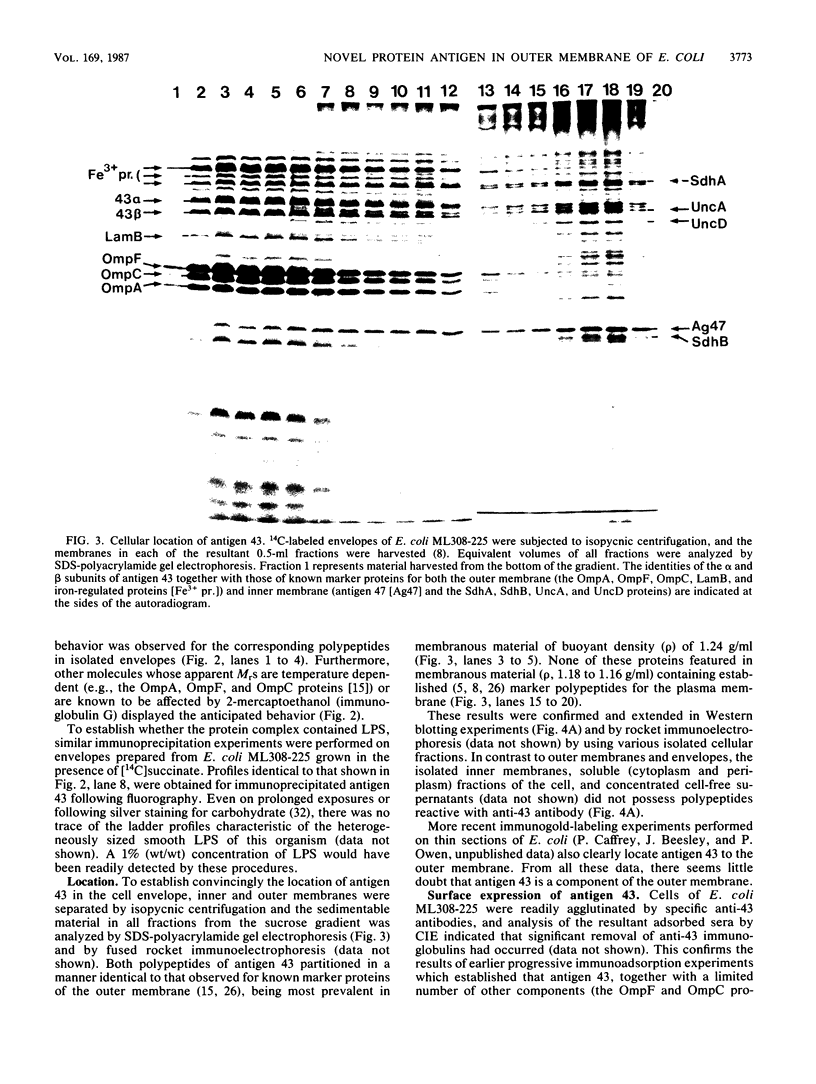
A study by crossed immunoelectrophoresis performed in conjunction with precipitate excision and polypeptide analysis identified a new antigen complex in the envelope of Escherichia coli ML308-225. This antigen corresponds to antigen 43 in the crossed immunoelectrophoresis profile of membrane vesicles (P. Owen and H. R. Kaback, Proc. Natl. Acad. Sci. USA 75:3148-3152, 1978). Immunoprecipitation experiments conducted with specific antiserum revealed that the complex was expressed on the cell surface and that it contained, in equal stoichiometry, two chemically distinct polypeptides termed alpha and beta (Mrs of 60,000 and 53,000, respectively). The beta polypeptide was heat modifiable, displaying an apparent Mr of 37,000 when solubilized at temperatures below 70 degrees C. Analysis of fractions obtained following cell disruption, isopycnic centrifugation, and detergent extraction indicated that both alpha and beta polypeptides were components of the outer membrane. The two polypeptides were not linked by disulfide bonds, and neither was peptidoglycan associated. The complex contained no detectable lipopolysaccharide, enzyme activity, fatty acyl groups, or other cofactors. Neither correlated with E. coli proteins of similar molecular weight which had previously been shown to be associated with the outer membrane. Antibodies were raised to individual alpha and beta polypeptides. Each of these sera was shown to be subunit specific when tested against denatured membrane proteins. In contrast, each immunoglobulin preparation coprecipitated both alpha and beta polypeptides when tested against undenatured proteins derived from Triton X-100-treated membranes. The results reveal the presence of a novel bipartite protein antigen in the outer membrane of E. coli.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R. Porin from bacterial and mitochondrial outer membranes. CRC Crit Rev Biochem. 1985;19(2):145–190. doi: 10.3109/10409238509082542. [DOI] [PubMed] [Google Scholar]
- Bowles L. K., Miguel A. G., Konisky J. Purification of the colicin I receptor. J Biol Chem. 1983 Jan 25;258(2):1215–1220. [PubMed] [Google Scholar]
- Condon C., Cammack R., Patil D. S., Owen P. The succinate dehydrogenase of Escherichia coli. Immunochemical resolution and biophysical characterization of a 4-subunit enzyme complex. J Biol Chem. 1985 Aug 5;260(16):9427–9434. [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Fine structure and isolation of the hook-basal body complex of flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):384–395. doi: 10.1128/jb.105.1.384-395.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Purification of intact flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):376–383. doi: 10.1128/jb.105.1.376-383.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty H., Yamada H., Caffrey P., Owen P. Identification, immunochemical characterization, and purification of a major lipoprotein antigen associated with the inner (cytoplasmic) membrane of Escherichia coli. J Bacteriol. 1986 Jun;166(3):1072–1082. doi: 10.1128/jb.166.3.1072-1082.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Fiss E. H., Hollifield W. C., Jr, Neilands J. B. Absence of ferric enterobactin receptor modification activity in mutants of Escherichia coli K-12 lacking protein a. Biochem Biophys Res Commun. 1979 Nov 14;91(1):29–34. doi: 10.1016/0006-291x(79)90578-3. [DOI] [PubMed] [Google Scholar]
- Ichihara S., Mizushima S. Arrangement of proteins O-8 and O-9 in outer membrane of Escherichia coli K-12. Existence of homotrimers and heterotrimers. Eur J Biochem. 1979 Oct 15;100(2):321–328. doi: 10.1111/j.1432-1033.1979.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Kadner R. J. Repression of synthesis of the vitamin B12 receptor in Escherichia coli. J Bacteriol. 1978 Dec;136(3):1050–1057. doi: 10.1128/jb.136.3.1050-1057.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R., Manning P. A., Reeves P. Identification and characterization of the TolC protein, an outer membrane protein from Escherichia coli. J Bacteriol. 1983 Feb;153(2):693–699. doi: 10.1128/jb.153.2.693-699.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Sawyer W. D., Haak R. A. Cross-linking analysis of the outer membrane proteins of Neisseria gonorrhoeae. Infect Immun. 1980 Jun;28(3):785–791. doi: 10.1128/iai.28.3.785-791.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima M., Nakaike S., Tamori Y., Nojima S. Detergent-resistant phospholipase A of Escherichia coli K-12. Purification and properties. Eur J Biochem. 1977 Feb 15;73(1):115–124. doi: 10.1111/j.1432-1033.1977.tb11297.x. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Molecular structure of membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3148–3152. doi: 10.1073/pnas.75.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Josefsson L. G. Precursors of three exported proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1209–1212. doi: 10.1073/pnas.75.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Purification and properties of the colicin E3 receptor of Escherichia coli. J Biol Chem. 1973 Mar 10;248(5):1797–1806. [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Swanson J. Surface-exposed protein antigens of the gonococcal outer membrane. Infect Immun. 1981 Dec;34(3):804–816. doi: 10.1128/iai.34.3.804-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Wolfe P. B., Silver P., Wickner W. The isolation of homogeneous leader peptidase from a strain of Escherichia coli which overproduces the enzyme. J Biol Chem. 1982 Jul 10;257(13):7898–7902. [PubMed] [Google Scholar]
- Zaleska M., Lounatmaa K., Nurminen M., Wahlström E., Mäkelä P. H. A novel virulence-associated cell surface structure composed of 47-kd protein subunits in Yersinia enterocolitica. EMBO J. 1985 Apr;4(4):1013–1018. doi: 10.1002/j.1460-2075.1985.tb03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]