Abstract
Background
Airway colonization of mechanically ventilated very low birth weight infants (birth weight < 1500 grams) by Ureaplasma urealyticum (Uu) is associated with an increased risk of bronchopulmonary dysplasia (BPD). While Uu is sensitive to erythromycin in vitro, the efficacy of intravenous (IV) erythromycin to eliminate Uu from the airways has not been studied.
Methods
17 very low birth weight infants with Uu positive tracheal aspirate (TA) cultures were randomized to either 5 (8 infants) or 10 days (9 infants) of IV erythromycin lactobionate (40 mg/kg/day in 3 divided doses). Tracheal aspirate cultures for Uu were performed on days 0, 5, 10 and 15.
Results
Intravenous erythromycin failed to eliminate airway colonization in a large proportion of infants regardless of whether they received 5 or 10 days of treatment. Ureaplasma urealyticum was isolated from 4/15 (27%) of TAs obtained at 5 days, 5/12 TAs (42%) obtained at 10 days and 6/11(55%) TAs obtained at 15 days (combined group data).
Conclusions
Erythromycin administered IV does not eliminate Uu from the airways in a large proportion of infants. Failure of erythromycin to eliminate Uu from the airways may contribute to the lack of efficacy of this drug in reducing the incidence of BPD in very low birth weight infants.
Background
Airway colonization or infection with Ureaplasma urealyticum (Uu) has been associated with an increased inflammatory response and subsequent development of chronic lung disease in mechanically ventilated very low birth weight infants [1-6]. Eradication of these organisms could potentially reduce lung injury and reduce the incidence of chronic lung disease. While most Uu isolates are sensitive to macrolide antibiotics in vitro, the clinical efficacy of erythromycin to clear these organisms from the respiratory tract has not been documented extensively [5,7-9]. Treatment of Uu infections with erythromycin in mechanically ventilated very low birth weight infants has failed to reduce the incidence of chronic lung disease [10-12]. The lack of effect of erythromycin on the incidence of chronic lung disease may be a result of failure to eliminate Uu from the lung. In order to address these questions, we designed a pilot study to examine the efficacy of either a 5 or 10 day course of intravenous erythromycin to eradicate Uu from the airways of very low birth weight infants.
Methods
The study population consisted of 17 very low birth weight (VLBW) infants admitted to the neonatal intensive care unit (NICU) at the Louisiana State University Health Sciences Center (LSUHSC) in Shreveport between May 1998 and July 2001. We designed a randomized, prospective, non-blinded evaluation of the efficacy of 5 versus 10 days of intravenous (IV) erythromycin lactobionate to eradicate Uu from the airways of mechanically ventilated VLBW infants. Mechanically ventilated VLBW infants were cultured for Uu in the airways (screening TA culture) at the discretion of the attending neonatologists. Infants who were still intubated and whose physician decided to treat with erythromycin were eligible for study. Infants were excluded from the study if they were moribund and not expected to survive, or if consent to participate was refused by the parents.
Randomization was performed by the use of sealed envelopes with the allocation of patients to either a 5 day or 10 day course of intravenous erythromycin lactobionate (40 mg/kg/day). Randomization strategy utilized blocks of 10, with 5 cards assigned to each treatment group. There was no stratification by weight or gestation. Tracheal aspirates were cultured for the presence of Uu on the day that treatment was initiated (Day 0) and on the 5th, 10th and 15th day thereafter if the infant remained intubated. The study was approved by the Institutional Review Board for Human Research at LSUHSC-Shreveport.
Tracheal aspirates were collected, transported to the lab and cultured for Uu on the same day. Fifty microliters of tracheal aspirate was placed in 2 ml of SP4 with urea (Remel, Lexana, KS), urea enriched PPLO broths and Mycotrim GU® biphasic culture system (Irvine Scientific, Santa Anna, CA) [13]. The media was inspected twice per day for the first 2 days, and then inspected daily for 5 days to observe any color change in the media indicative of a positive culture. The presence of Uu was determined by color change in the broth and microscopic observation of characteristic colony morphology. Follow up cultures were performed using SP4 with urea and urea enriched PPLO broths.
The primary endpoint of this study was the eradication of Uu from the airways of the infant. Originally it was planned to study 30 infants (15 patients in each group). The study was terminated early when it became apparent that there was an unacceptably high incidence of treatment failure in both groups. Infants were followed through their initial hospitalization and information on respiratory outcomes was collected.
Statistical analysis was performed using the SPSS for Windows version 6.0 (SPSS Inc., Chicago, IL). Fisher's Exact test was used to assess the statistical differences in categorical variables. The Mann-Whitney U test was used to assess significance of continuous variables. A probability value of less than 0.05 was considered statistically significant. The data are presented as median and (range).
Results
There were 17 infants enrolled in the study. Nine patients received 10 days of treatment and 8 patients received 5 days. Patients were enrolled in the study at 7 (2–33) days. The clinical characteristics of the patients are shown in Table 1. There were no differences in birth weight, gestational age, pre and post-natal steroid exposure or age of enrollment in the study between infants who were treated for 5 days as compared to those treated for 10 days (Table 2). One infant (randomized to receive 5 days of erythromycin) had a Day 0 TA culture that was negative for Uu.
Table 1.
Clinical Characteristics of Subjects (All Patients) N = 17
| Gestation (weeks) | 25 (23–29) |
| Birth Weight (grams) | 777 (620–1160) |
| Histological Chorioamnionitis* | 6/11 (55) |
| Prenatal Steroids | 11/17 (65) |
| RDS | 16/17 (94) |
| Surfactant Therapy | 16/17 (94) |
| Postnatal Steroids | 15/17 (88) |
| Duration Oxygen Therapy (days) | 64 ± 4 |
| Duration Mechanical Ventilation (days) | 31 ± 4 |
| Oxygen at 28 days | 13/17 (77) |
| Oxygen at 36 weeks PCA | 4/17 (24) |
| IVH | 4/17 (24) |
Data are presented as Median (range). Numbers in parenthesis are percentages RDS Respiratory Distress Syndrome IVH Intraventricular hemorrhage PCA Postconceptional age *Not all patients had pathological examination of the placenta
Table 2.
Clinical Characteristics of Subjects
| 5 days n = 8 | 10 days n = 9 | p | |
| Birth weight | 810 (775–965) | 720 (620–1160) | 0.177 |
| Gestation | 25 (24–29) | 25 (23–28) | 0.921 |
| ROM (hours) | 5.25 (0–70) | 30 (0–504) | 0.514 |
| Prenatal Steroids | 5 (63) | 6 (66) | 1.000 |
| Histologic Chorioamnionitis* | 1 (33) | 5 (55) | 0.545 |
| Age at Enrollment | 8 (3–11) | 7 (2–33) | 0.529 |
| Surfactant therapy | 8 (100) | 8 (88) | 1.000 |
| Postnatal Steroids | 6 (75) | 9 (100) | 0.206 |
Data are presented as Median (range). Numbers in parenthesis are percentages *Not all patients had pathological examination of the placenta
Erythromycin failed to eradicate Uu from the airways in a significant proportion of infants who were treated for either 5 or 10 days (Table 3). There were no significant differences in the proportion of infants in whom Uu was successfully eliminated from the airways between those who were treated for 5 days compared to 10 days. Overall, 6 of the 11 (55%) infants who were re-cultured after 14 days had positive airway cultures for Uu. The pattern of positive Uu cultures varied considerably from patient to patient. Some patients cleared the Uu while on erythromycin only to have positive cultures once treatment was stopped. Others were persistently positive for Uu throughout the study, while others cleared after a period of treatment with erythromycin and remained free of Uu.
Table 3.
Follow Up TA Cultures for Ureaplasma urealyticum
| Percent Positive Uu | ||||
| Group | Day 0 | Day 5 | Day 10 | Day 15 |
| 5 Days (n = 8) | 7/8 (88%)* | 3/7 (43%) | 3/4 (75%) | 3/4 (75%) |
| 10 Days (n = 9) | 9/9 (100%) | 1/8 (13%) | 2/8 (25%) | 3/7 (43%) |
| All patients | 16/17 (94%) | 4/15 (27%) | 5/12 (42%) | 6/10 (55%) |
* One infant who a positive screening culture for Ureaplasma urealyticum had a negative culture on Day 0 when starting therapy. A follow up culture on Day 5 again was positive for Uu.
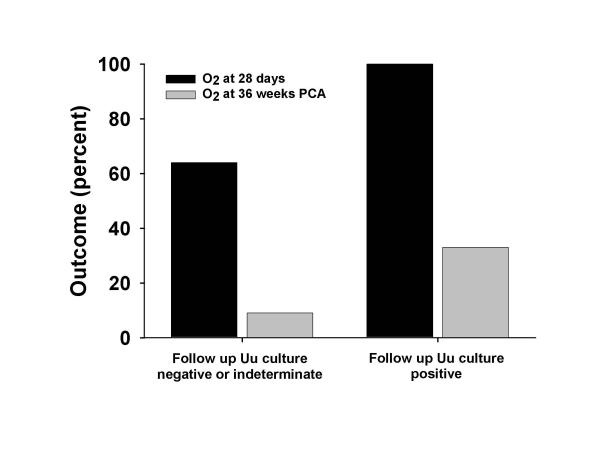
Infants who had persistent airway colonization with Ureaplasma urealyticum after completion of erythromycin therapy had increased duration of mechanical ventilation (42 (24–73) vs. 24 (9–52) days; p = 0.035) and increased duration of oxygen dependency (106 (44–200) vs. 41 (13–97) days; p = 0.016). There was a trend towards an increased incidence of chronic lung disease in infants with persistent Ureaplasma in the airways (Figure 1).
Figure 1.
Incidence of chronic lung disease in infants with positive follow up cultures for Ureaplasma urealyticum after completing intravenous erythromycin lactobionate. There were trends for increased incidence of oxygen dependency at 28 days of life (p = 0.091) and oxygen dependency at 36 weeks (p = 0.057) in infants with any positive culture for Uu after completing therapy. Dark bars represent the incidence of oxygen dependency at 28 days of life. White bars represent the incidence of oxygen dependency at 36 weeks postconceptional age.
Discussion
Colonization and/or infection with Uu have been associated with an increased risk of developing chronic lung disease in several studies [1-4]. Because this finding has not been confirmed by other studies, the causal link between Uu and chronic lung disease remains controversial. Despite this controversy, more than 50% of neonatologists surveyed indicated they would provide antibiotic therapy to preterm infants with respiratory disease who had Uu positive tracheal (or even pharyngeal) cultures [14]. The decision to culture infants and whether to treat positive cultures were made by the attending physicians based on the clinical situation. Thus not all VLBW infants were cultured and not all infants with positive cultures were treated. This selection bias may influence the interpretation of the results as the tendency is to treat smaller, sicker infants. However, the purpose of this study was not to determine if treatment prevents the development of BPD but to assess if erythromycin treatment could eradicate Uu from the airways. This is particularly relevant in view of the recent report correlating persistence of Uu with the development of chronic lung disease. [15]. In the 11 infants who remained intubated for repeat cultures, 54% were still positive. Because of this finding, we believed that continued exposure of infants to the toxicities and risks of the trial were not warranted. This obviously limits many of the conclusions that could be drawn. It is possible that erythromycin therapy may be more efficacious than is presented in this small study. However, we do believe that the real failure rate of erythromycin is too high for it to be useful in this setting.
Clinical isolates of Uu are susceptible to macrolide antibiotics, tetracyclines, quinolones and chloramphenicol [5,7-9]. Erythromycin, a macrolide antibiotic, has been suggested as the drug of choice to treat neonatal Uu infections due to unacceptable side effects of the other potential agents [5]. Four clinical studies (2 randomized controlled trials) have evaluated the use of erythromycin to treat Uu infected/colonized infants of which 2 of the studies evaluated microbiological outcome [10-12,16]. Treatment with erythromycin did not affect the incidence of chronic lung disease in any of these trials. In our study, intravenous erythromycin lactobionate (40 mg/kg/day) failed to eradicate Uu in greater than 50% of colonized very low birth weight infants. This contrasts with the finding of two earlier studies in which erythromycin eliminated Uu colonization in 15/19 (79%) and 12/14 (86%) infants treated [11,16]. There are several potential reasons that might explain the differences observed. In the study reported by Bowman, the timing of repeat cultures was not defined [16]. It is possible that follow up cultures were taken shortly after completing therapy when residual erythromycin may have suppressed the growth of Uu. This is not likely in Jonsson's study in which follow up cultures were performed a week after completing therapy [11]. We utilized a similar or higher dose of erythromycin compared to the previous studies, making drug dose an unlikely factor in the failure of erythromycin to eliminate Uu.
Isolation of Uu with resistance or intermediate sensitivity to erythromycin has been reported [8,9,17]. Depending on the methodology employed, the rate of intermediate sensitivities (MIC 1 – 4 μg/ml) to erythromycin may be as high as 100% [9]. Peak serum concentrations of erythromycin following 40 mg/kg/day intravenous dosing are approximately 3.4 μg/ml [18]. Although data in neonates are lacking, erythromycin concentrations in bronchial secretions are less than 25% of serum concentrations in adults [19]. Therefore the local concentration of erythromycin in the airways/lung may not be sufficient to eradicate Uu with intermediate antibiotic sensitivities. We did not measure erythromycin sensitivities in our isolates.
Anti-microbial treatment of Uu colonization or infection has not proven successful in preventing the development of chronic lung disease. We believe the high failure rate of IV erythromycin in eliminating Uu from the airway that we observed in this study may underlie the failure of erythromycin therapy to reduce the incidence of chronic lung disease. In our study, infants who failed to clear Uu were ventilated longer and were at increased risk of chronic lung disease, suggesting a role for prolonged infection or colonization with adverse outcome.
In summary we found that treatment of Uu colonization with intravenous erythromycin was associated with a high failure rate in our NICU population. Treatment strategies with other agents may be required to eliminate Uu from the airways of colonized preterm infants in order to minimize the incidence of chronic lung disease.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
R John Baier, Email: jbaier@lsuhsc.edu.
John Loggins, Email: jloggi@lsuhsc.edu.
Thomas E Kruger, Email: dkruger1@kc.rr.net.
References
- Cassell GH, Waites KB, Crouse DT, Rudd PT, Canupp KC, Stagno S, Cutter GR. Association of Ureaplasma urealyticum infection of the lower respiratory tract with chronic lung disease and death in very-low-birth-weight infants. Lancet. 1988;2:240–245. doi: 10.1016/S0140-6736(88)92536-6. [DOI] [PubMed] [Google Scholar]
- Wang EE, Frayha H, Watts J, Hammerberg O, Chernesky MA, Mahony JB, Cassell GH. Role of Ureaplasma urealyticum and other pathogens in the development of chronic lung disease of prematurity. Pediatr Infect Dis J. 1988;7:547–551. [PubMed] [Google Scholar]
- Wang EE, Ohlsson A, Kellner JD. Association of Ureaplasma urealyticum colonization with chronic lung disease of prematurity: results of a metaanalysis. J Pediatr. 1995;127:640–644. doi: 10.1016/s0022-3476(95)70130-3. [DOI] [PubMed] [Google Scholar]
- Baier RJ, Loggins J, Kruger TE. Monocyte chemoattractant protein-1 and interleukin-8 are increased in bronchopulmonary dysplasia: relation to isolation of Ureaplasma urealyticum. J Investig Med. 2001;49:362–369. doi: 10.2310/6650.2001.33902. [DOI] [PubMed] [Google Scholar]
- Waites KB, Crouse DT, Cassell GH. Antibiotic susceptibilities and therapeutic options for Ureaplasma urealyticum infections in neonates. Pediatr Infect Dis J. 1992;11:23–29. doi: 10.1097/00006454-199201000-00007. [DOI] [PubMed] [Google Scholar]
- Patterson AM, Taciak V, Lovchik J, Fox RE, Campbell AB, Viscardi RM. Ureaplasma urealyticum respiratory tract colonization is associated with an increase in interleukin 1-beta and tumor necrosis factor alpha relative to interleukin 6 in tracheal aspirates of preterm infants. Pediatr Infect Dis J. 1998;17:321–328. doi: 10.1097/00006454-199804000-00011. [DOI] [PubMed] [Google Scholar]
- Matlow A, Th'ng C, Kovach D, Quinn P, Dunn M, Wang E. Susceptibilities of neonatal respiratory isolates of Ureaplasma urealyticum to antimicrobial agents. Antimicrob Agents Chemother. 1998;42:1290–1292. doi: 10.1128/aac.42.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudin H, Bebear C. Comparative in vitro activity of azithromycin, clarithromycin, erythromycin and lomefloxacin against Mycoplasma pneumoniae, Mycoplasma hominis and Ureaplasma urealyticum. Eur J Clin Microbiol Infect Dis. 1990;9:838–841. doi: 10.1007/BF01967388. [DOI] [PubMed] [Google Scholar]
- Renaudin H, Bebear C, Robertson JA. In vitro susceptibility of tetracycline-resistant strains of Ureaplasma urealyticum to newer macrolides and quinolones, and a streptogramin. Eur J Clin Microbiol Infect Dis. 1991;10:984–986. doi: 10.1007/BF02005461. [DOI] [PubMed] [Google Scholar]
- Lyon AJ, McColm J, Middlemist L, Fergusson S, McIntosh N, Ross PW. Randomised trial of erythromycin on the development of chronic lung disease in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;78:F10–4. doi: 10.1136/fn.78.1.f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson B, Rylander M, Faxelius G. Ureaplasma urealyticum, erythromycin and respiratory morbidity in high-risk preterm neonates. Acta Paediatr. 1998;87:1079–1084. doi: 10.1080/080352598750031428. [DOI] [PubMed] [Google Scholar]
- Heggie AD, Jacobs MR, Butler VT, Baley JE, Boxerbaum B. Frequency and significance of isolation of Ureaplasma urealyticum and Mycoplasma hominis from cerebrospinal fluid and tracheal aspirate specimens from low birth weight infants. J Pediatr. 1994;124:956–961. doi: 10.1016/s0022-3476(05)83192-0. [DOI] [PubMed] [Google Scholar]
- Isenberg Henry D., American Society for Microbiology. Clinical microbiology procedures handbook. Washington, D.C., American Society of Microbiology; 1992. p. 2 v. (various pagings). [Google Scholar]
- Taylor RS. Postnatal steroid use among neonatologists: Survey results. http://www.cheo.on.ca/rtaylor/PNSteroid44.htm.
- Castro-Alcaraz S, Greenberg EM, Bateman DA, Regan JA. Patterns of colonization with Ureaplasma urealyticum during neonatal intensive care unit hospitalizations of very low birth weight infants and the development of chronic lung disease. Pediatrics. 2002;110:e45. doi: 10.1542/peds.110.4.e45. [DOI] [PubMed] [Google Scholar]
- Bowman ED, Dharmalingam A, Fan WQ, Brown F, Garland SM. Impact of erythromycin on respiratory colonization of Ureaplasma urealyticum and the development of chronic lung disease in extremely low birth weight infants. Pediatr Infect Dis J. 1998;17:615–620. doi: 10.1097/00006454-199807000-00008. [DOI] [PubMed] [Google Scholar]
- Palu G, Valisena S, Barile MF, Meloni GA. Mechanisms of macrolide resistance in Ureaplasma urealyticum: a study on collection and clinical strains. Eur J Epidemiol. 1989;5:146–153. doi: 10.1007/BF00156820. [DOI] [PubMed] [Google Scholar]
- Waites KB, Sims PJ, Crouse DT, Geerts MH, Shoup RE, Hamrick WB, Duffy LB, Cassell GH. Serum concentrations of erythromycin after intravenous infusion in preterm neonates treated for Ureaplasma urealyticum infection. Pediatr Infect Dis J. 1994;13:287–293. doi: 10.1097/00006454-199404000-00008. [DOI] [PubMed] [Google Scholar]
- Pierre J, Bergogne-Berezin E, Kafe H, Dournovo P. The penetration of macrolides into bronchial secretions. J Antimicrob Chemother. 1985;16 Suppl A:217–220. doi: 10.1093/jac/16.suppl_a.217. [DOI] [PubMed] [Google Scholar]