Abstract
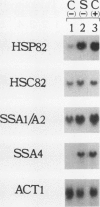
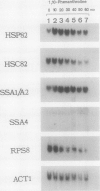
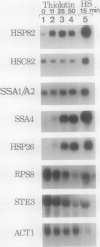
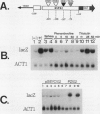
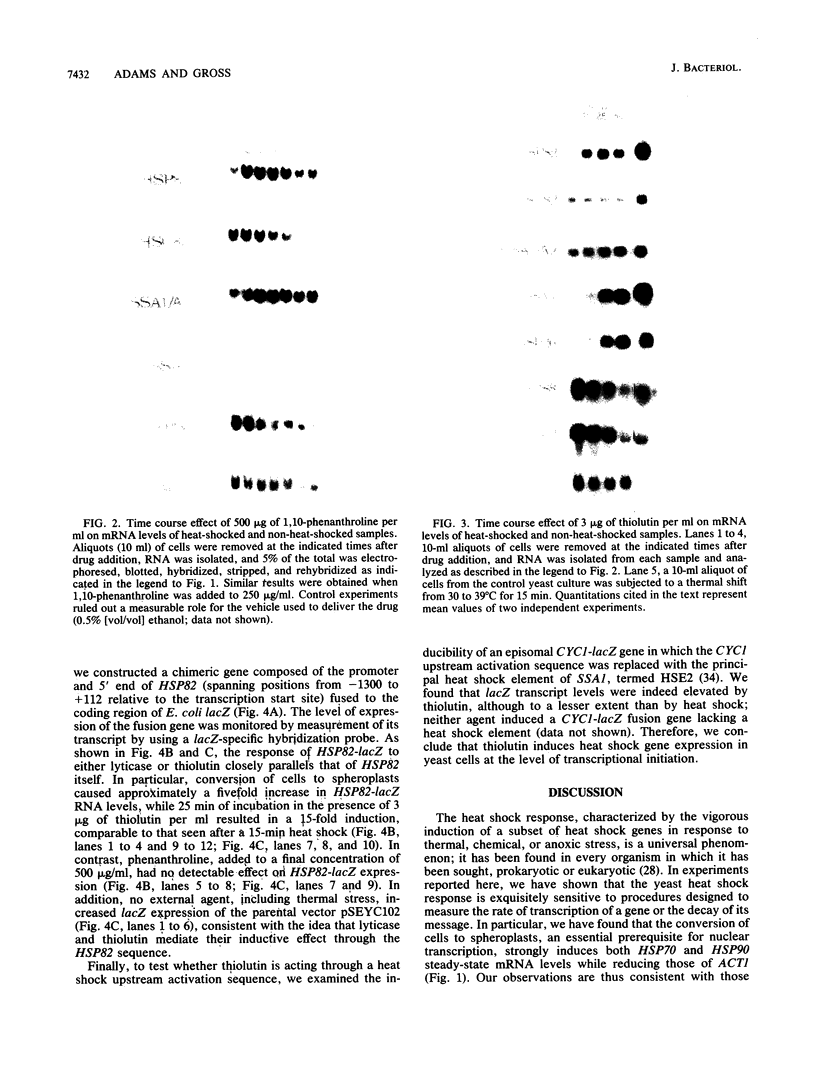
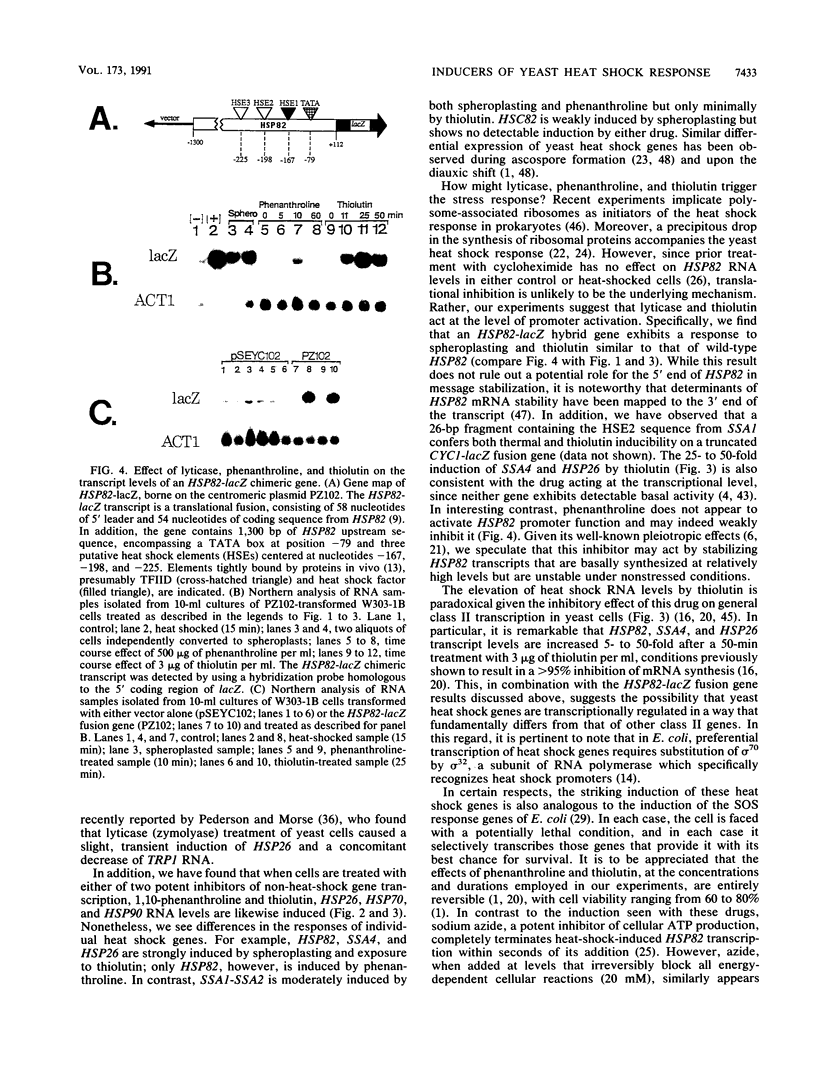
We report here that procedures commonly used to measure transcription and mRNA decay rates in Saccharomyces cerevisiae induce the heat shock response. First, conversion of cells to spheroplasts with lyticase, a prerequisite for nuclear runoff transcription, induces the expression of HSP70 and HSP90 heat shock genes. The transcript levels of the non-heat-shock gene ACT1 are slightly depressed, consistent with the general yeast stress response. Second, the DNA intercalator, 1,10-phenanthroline, widely employed as a general transcriptional inhibitor in S. cerevisiae, enhances the mRNA abundance of certain heat shock genes (HSP82, SSA1-SSA2) although not of others (HSC82, SSA4, HSP26). Third, the antibiotic thiolutin, previously demonstrated to inhibit all three yeast RNA polymerases both in vivo and in vitro, increases the RNA levels of HSP82 5- to 10-fold, those of SSA4 greater than 25-fold, and those of HSP26 greater than 50-fold under conditions in which transcription of non-heat-shock genes is blocked. By using an episomal HSP82-lacZ fusion gene, we present evidence that lyticase and thiolutin induce heat shock gene expression at the level of transcription, whereas phenanthroline acts at a subsequent step, likely through message stabilization. We conclude that, because of the exquisite sensitivity of the yeast heat shock response, procedures designed to measure the rate of gene transcription or mRNA turnover can themselves impact upon each process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Boorstein W. R., Craig E. A. Structure and regulation of the SSA4 HSP70 gene of Saccharomyces cerevisiae. J Biol Chem. 1990 Nov 5;265(31):18912–18921. [PubMed] [Google Scholar]
- Borkovich K. A., Farrelly F. W., Finkelstein D. B., Taulien J., Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989 Sep;9(9):3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. J. Messenger RNA stability in yeast. Yeast. 1989 Jul-Aug;5(4):239–257. doi: 10.1002/yea.320050405. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Percival K. J. Transformation of yeast spheroplasts without cell fusion. Anal Biochem. 1987 Jun;163(2):391–397. doi: 10.1016/0003-2697(87)90240-5. [DOI] [PubMed] [Google Scholar]
- Drew H. R. Structural specificities of five commonly used DNA nucleases. J Mol Biol. 1984 Jul 15;176(4):535–557. doi: 10.1016/0022-2836(84)90176-1. [DOI] [PubMed] [Google Scholar]
- Farrelly F. W., Finkelstein D. B. Complete sequence of the heat shock-inducible HSP90 gene of Saccharomyces cerevisiae. J Biol Chem. 1984 May 10;259(9):5745–5751. [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Lebowitz P., Weissman S. M. Heterogeneity and 5'-terminal structures of the late RNAs of simian virus 40. J Mol Biol. 1978 Dec 25;126(4):813–846. doi: 10.1016/0022-2836(78)90022-0. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Gross D. S., Collins K. W., Hernandez E. M., Garrard W. T. Vacuum blotting: a simple method for transferring DNA from sequencing gels to nylon membranes. Gene. 1988 Dec 30;74(2):347–356. doi: 10.1016/0378-1119(88)90168-0. [DOI] [PubMed] [Google Scholar]
- Gross D. S., English K. E., Collins K. W., Lee S. W. Genomic footprinting of the yeast HSP82 promoter reveals marked distortion of the DNA helix and constitutive occupancy of heat shock and TATA elements. J Mol Biol. 1990 Dec 5;216(3):611–631. doi: 10.1016/0022-2836(90)90387-2. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Erickson J. W., Gross C. A. The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell. 1984 Sep;38(2):383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Guarente L., Ptashne M. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2199–2203. doi: 10.1073/pnas.78.4.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick D., Parker R., Jacobson A. Identification and comparison of stable and unstable mRNAs in Saccharomyces cerevisiae. Mol Cell Biol. 1990 May;10(5):2269–2284. doi: 10.1128/mcb.10.5.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herruer M. H., Mager W. H., Raué H. A., Vreken P., Wilms E., Planta R. J. Mild temperature shock affects transcription of yeast ribosomal protein genes as well as the stability of their mRNAs. Nucleic Acids Res. 1988 Aug 25;16(16):7917–7929. doi: 10.1093/nar/16.16.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen B. K., Pelham H. R. Constitutive binding of yeast heat shock factor to DNA in vivo. Mol Cell Biol. 1988 Nov;8(11):5040–5042. doi: 10.1128/mcb.8.11.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome J. F., Jaehning J. A. mRNA transcription in nuclei isolated from Saccharomyces cerevisiae. Mol Cell Biol. 1986 May;6(5):1633–1639. doi: 10.1128/mcb.6.5.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A., Tipper D. J., Davies J. Mode of action of thiolutin, an inhibitor of macromolecular synthesis in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1973 Jun;3(6):729–738. doi: 10.1128/aac.3.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A. RNA synthesis and control of cell division in the yeast S. cerevisiae. Cell. 1978 Aug;14(4):951–958. doi: 10.1016/0092-8674(78)90349-5. [DOI] [PubMed] [Google Scholar]
- Kim C. H., Warner J. R. Mild temperature shock alters the transcription of a discrete class of Saccharomyces cerevisiae genes. Mol Cell Biol. 1983 Mar;3(3):457–465. doi: 10.1128/mcb.3.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz S., Rossi J., Petko L., Lindquist S. An ancient developmental induction: heat-shock proteins induced in sporulation and oogenesis. Science. 1986 Mar 7;231(4742):1154–1157. doi: 10.1126/science.3511530. [DOI] [PubMed] [Google Scholar]
- Larkin J. C., Thompson J. R., Woolford J. L., Jr Structure and expression of the Saccharomyces cerevisiae CRY1 gene: a highly conserved ribosomal protein gene. Mol Cell Biol. 1987 May;7(5):1764–1775. doi: 10.1128/mcb.7.5.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. S., Garrard W. T. Transcription-induced nucleosome 'splitting': an underlying structure for DNase I sensitive chromatin. EMBO J. 1991 Mar;10(3):607–615. doi: 10.1002/j.1460-2075.1991.tb07988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. RNA synthesis in isolated nuclei: in vitro initiation of adenovirus 2 major late mRNA precursor. Proc Natl Acad Sci U S A. 1979 Jan;76(1):160–164. doi: 10.1073/pnas.76.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Alterations in translatable ribonucleic acid after heat shock of Saccharomyces cerevisiae. J Bacteriol. 1980 Aug;143(2):603–612. doi: 10.1128/jb.143.2.603-612.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel D., Caplan A. J., Lee M. S., Adams C. C., Fishel B. R., Gross D. S., Garrard W. T. Basal-level expression of the yeast HSP82 gene requires a heat shock regulatory element. Mol Cell Biol. 1989 Nov;9(11):4789–4798. doi: 10.1128/mcb.9.11.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M., Scafe C., Sexton J., Young R. Eucaryotic RNA polymerase conditional mutant that rapidly ceases mRNA synthesis. Mol Cell Biol. 1987 May;7(5):1602–1611. doi: 10.1128/mcb.7.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. O., Craig E. A. Positive and negative regulation of basal expression of a yeast HSP70 gene. Mol Cell Biol. 1989 May;9(5):2025–2033. doi: 10.1128/mcb.9.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R., Herrick D., Peltz S. W., Jacobson A. Measurement of mRNA decay rates in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:415–423. doi: 10.1016/0076-6879(91)94032-8. [DOI] [PubMed] [Google Scholar]
- Pederson D. S., Morse R. H. Effect of transcription of yeast chromatin on DNA topology in vivo. EMBO J. 1990 Jun;9(6):1873–1881. doi: 10.1002/j.1460-2075.1990.tb08313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago T. C., Purvis I. J., Bettany A. J., Brown A. J. The relationship between mRNA stability and length in Saccharomyces cerevisiae. Nucleic Acids Res. 1986 Nov 11;14(21):8347–8360. doi: 10.1093/nar/14.21.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. H., Schekman R. Lyticase: endoglucanase and protease activities that act together in yeast cell lysis. J Bacteriol. 1980 May;142(2):414–423. doi: 10.1128/jb.142.2.414-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M. R., Craig E. A. The SSA1 and SSA2 genes of the yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1989 Jan 25;17(2):805–806. doi: 10.1093/nar/17.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek R. E., Lindquist S. Transcriptional derepression of the Saccharomyces cerevisiae HSP26 gene during heat shock. Mol Cell Biol. 1990 Dec;10(12):6362–6373. doi: 10.1128/mcb.10.12.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J. Inhibition of yeast ribonucleic acid polymerases by thiolutin. J Bacteriol. 1973 Oct;116(1):245–256. doi: 10.1128/jb.116.1.245-256.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Neidhardt F. C. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Washburne M., Becker J., Kosic-Smithers J., Craig E. A. Yeast Hsp70 RNA levels vary in response to the physiological status of the cell. J Bacteriol. 1989 May;171(5):2680–2688. doi: 10.1128/jb.171.5.2680-2688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]