Abstract
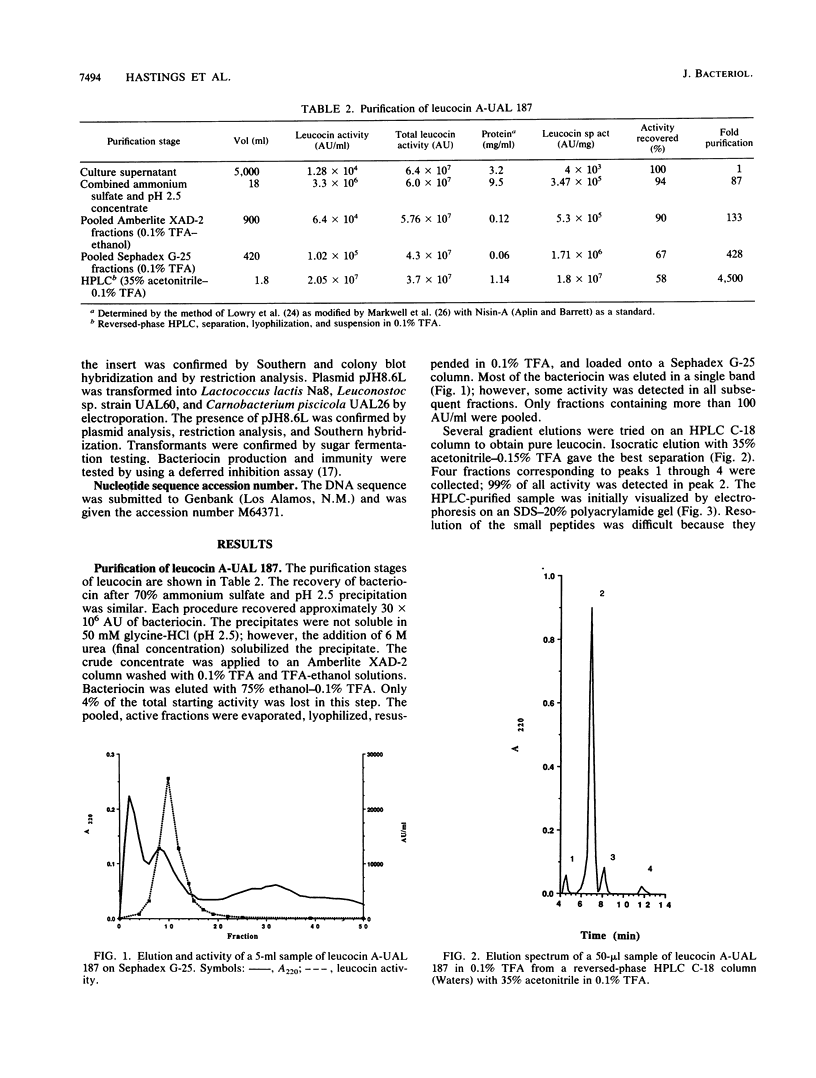
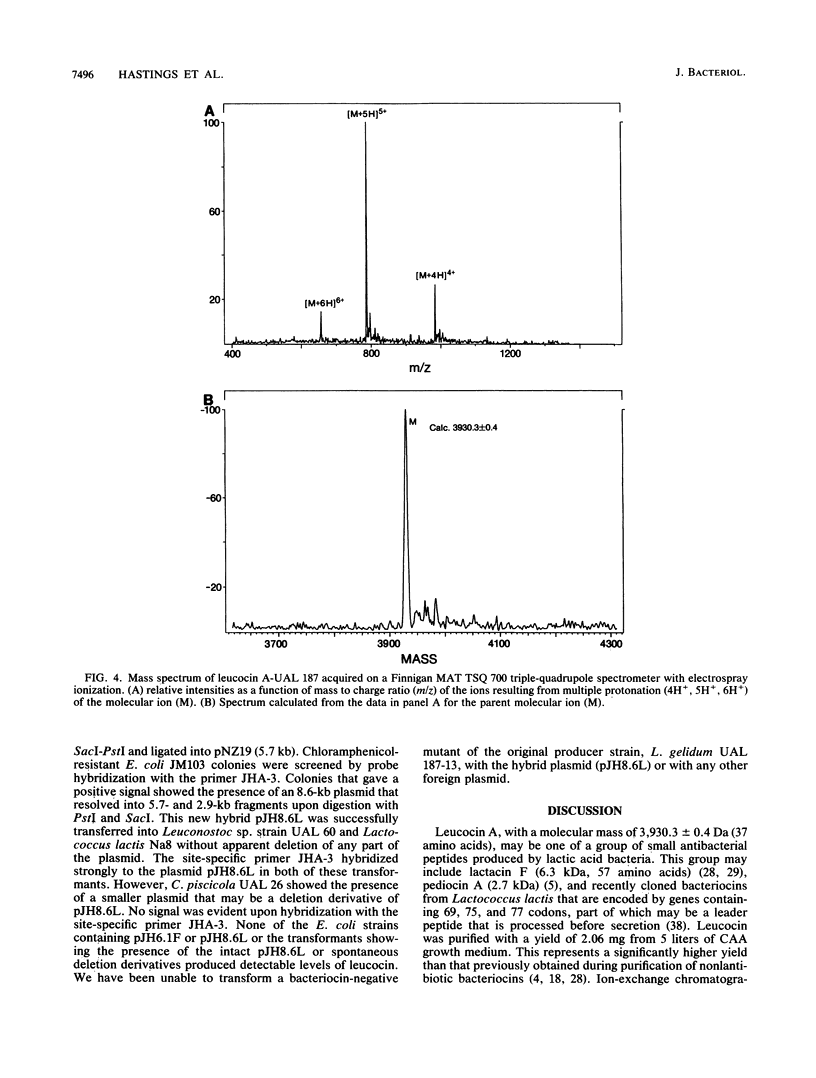
Leucocin A-UAL 187 is a bacteriocin produced by Leuconostoc gelidum UAL 187, a lactic acid bacterium isolated from vacuum-packaged meat. The bacteriocin was purified by ammonium sulfate or acid (pH 2.5) precipitation, hydrophobic interaction chromatography, gel filtration, and reversed-phase high-performance liquid chromatography with a yield of 58% of the original activity. Leucocin A is stable at low pH and heat resistant, and the activity of the pure form is enhanced by the addition of bovine serum albumin. It is inactivated by a range of proteolytic enzymes. The molecular weight was determined by mass spectrometry to be 3,930.3 +/- 0.4. Leucocin A-UAL 187 contains 37 amino acids with a calculated molecular weight of 3,932.3. A mixed oligonucleotide (24-mer) homologous to the sequence of the already known N terminus of the bacteriocin hybridized to a 2.9-kb HpaII fragment of a 7.6-MDa plasmid from the producer strain. The fragment was cloned into pUC118 and then subcloned into a lactococcal shuttle vector, pNZ19. DNA sequencing revealed an operon consisting of a putative upstream promoter, a downstream terminator, and two open reading frames flanked by a putative upstream promoter and a downstream terminator. The first open reading frame downstream of the promoter contains 61 amino acids and is identified as the leucocin structural gene, consisting of a 37-amino-acid bacteriocin and a 24-residue N-terminal extension. No phenotypic expression of the bacteriocin was evident in several lactic acid bacteria that were electrotransformed with pNZ19 containing the 2.9-kb cloned fragment of the leucocin A plasmid.
Full text
PDF



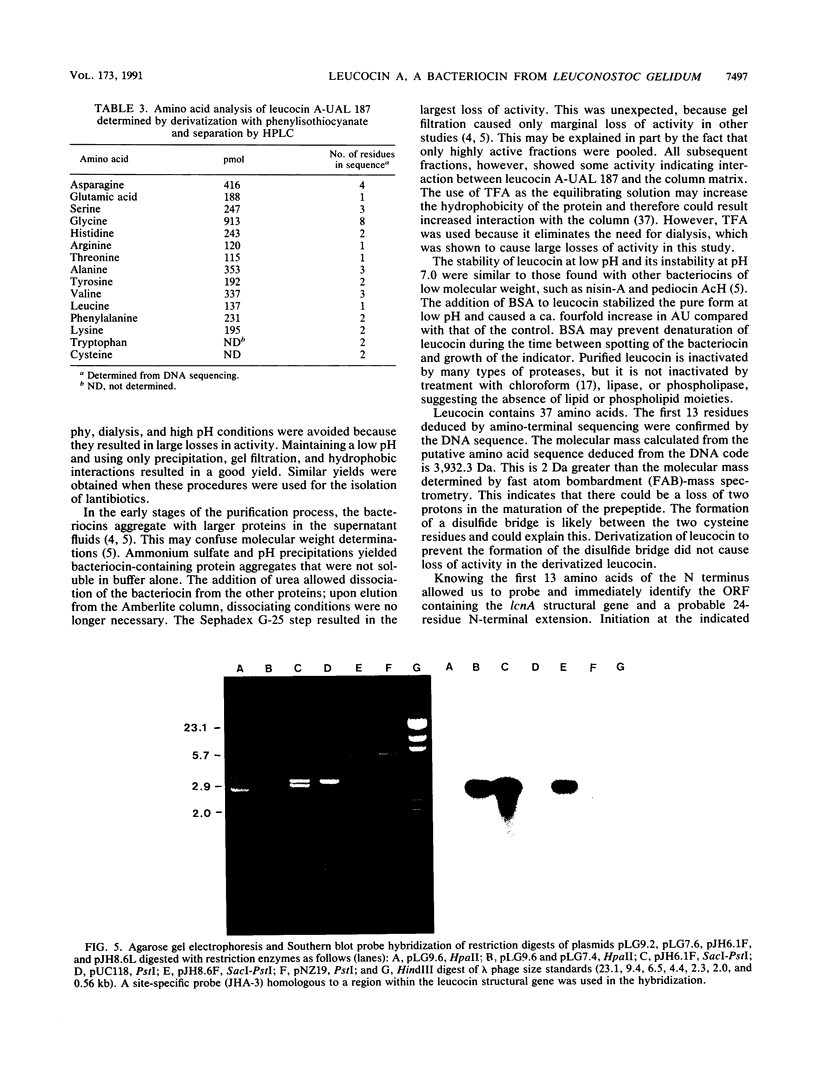

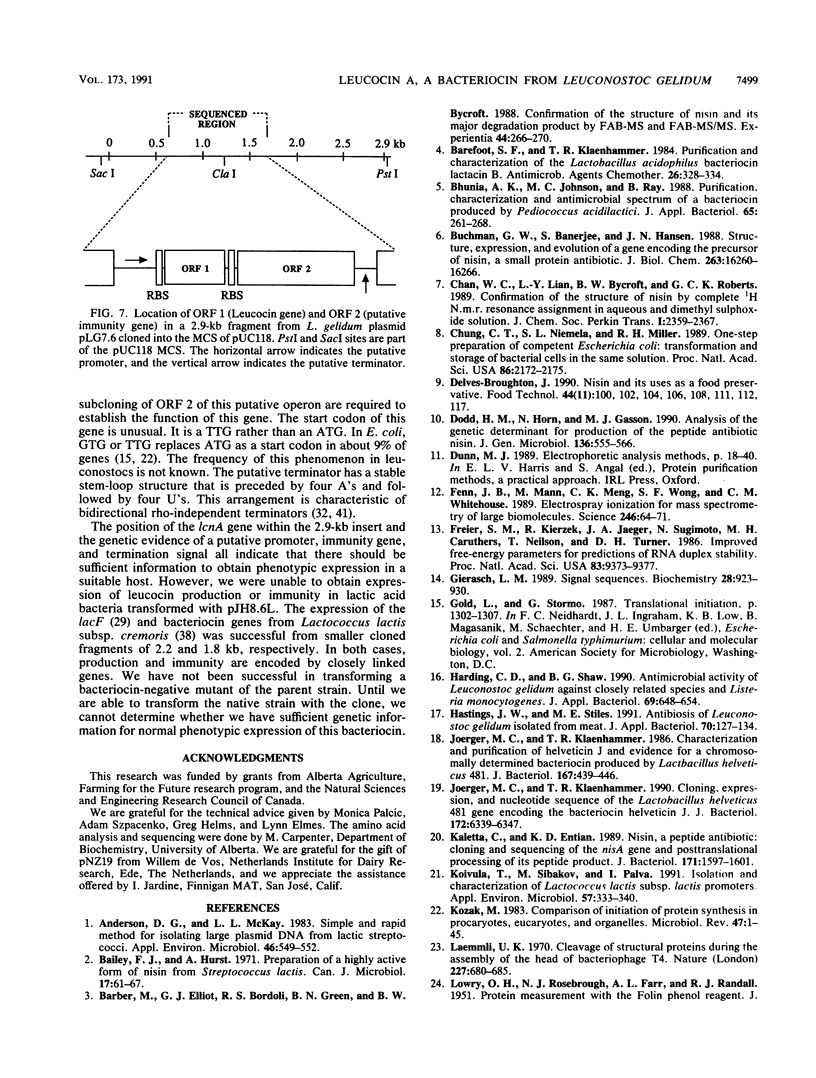

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey F. J., Hurst A. Preparation of a highly active form of nisin from Streptococcus lactis. Can J Microbiol. 1971 Jan;17(1):61–67. doi: 10.1139/m71-010. [DOI] [PubMed] [Google Scholar]
- Barber M., Elliot G. J., Bordoli R. S., Green B. N., Bycroft B. W. Confirmation of the structure of nisin and its major degradation product by FAB-MS and FAB-MS/MS. Experientia. 1988 Mar 15;44(3):266–270. doi: 10.1007/BF01941734. [DOI] [PubMed] [Google Scholar]
- Barefoot S. F., Klaenhammer T. R. Purification and characterization of the Lactobacillus acidophilus bacteriocin lactacin B. Antimicrob Agents Chemother. 1984 Sep;26(3):328–334. doi: 10.1128/aac.26.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhunia A. K., Johnson M. C., Ray B. Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J Appl Bacteriol. 1988 Oct;65(4):261–268. doi: 10.1111/j.1365-2672.1988.tb01893.x. [DOI] [PubMed] [Google Scholar]
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd H. M., Horn N., Gasson M. J. Analysis of the genetic determinant for production of the peptide antibiotic nisin. J Gen Microbiol. 1990 Mar;136(3):555–566. doi: 10.1099/00221287-136-3-555. [DOI] [PubMed] [Google Scholar]
- Fenn J. B., Mann M., Meng C. K., Wong S. F., Whitehouse C. M. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989 Oct 6;246(4926):64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Kierzek R., Jaeger J. A., Sugimoto N., Caruthers M. H., Neilson T., Turner D. H. Improved free-energy parameters for predictions of RNA duplex stability. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9373–9377. doi: 10.1073/pnas.83.24.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierasch L. M. Signal sequences. Biochemistry. 1989 Feb 7;28(3):923–930. doi: 10.1021/bi00429a001. [DOI] [PubMed] [Google Scholar]
- Harding C. D., Shaw B. G. Antimicrobial activity of Leuconostoc gelidum against closely related species and Listeria monocytogenes. J Appl Bacteriol. 1990 Nov;69(5):648–654. doi: 10.1111/j.1365-2672.1990.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Stiles M. E. Antibiosis of Leuconostoc gelidum isolated from meat. J Appl Bacteriol. 1991 Feb;70(2):127–134. doi: 10.1111/j.1365-2672.1991.tb04438.x. [DOI] [PubMed] [Google Scholar]
- Joerger M. C., Klaenhammer T. R. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. J Bacteriol. 1986 Aug;167(2):439–446. doi: 10.1128/jb.167.2.439-446.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger M. C., Klaenhammer T. R. Cloning, expression, and nucleotide sequence of the Lactobacillus helveticus 481 gene encoding the bacteriocin helveticin J. J Bacteriol. 1990 Nov;172(11):6339–6347. doi: 10.1128/jb.172.11.6339-6347.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletta C., Entian K. D. Nisin, a peptide antibiotic: cloning and sequencing of the nisA gene and posttranslational processing of its peptide product. J Bacteriol. 1989 Mar;171(3):1597–1601. doi: 10.1128/jb.171.3.1597-1601.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivula T., Sibakov M., Palva I. Isolation and characterization of Lactococcus lactis subsp. lactis promoters. Appl Environ Microbiol. 1991 Feb;57(2):333–340. doi: 10.1128/aem.57.2.333-340.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Cloning, phenotypic expression, and DNA sequence of the gene for lactacin F, an antimicrobial peptide produced by Lactobacillus spp. J Bacteriol. 1991 Mar;173(5):1779–1788. doi: 10.1128/jb.173.5.1779-1788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Purification and partial characterization of lactacin F, a bacteriocin produced by Lactobacillus acidophilus 11088. Appl Environ Microbiol. 1991 Jan;57(1):114–121. doi: 10.1128/aem.57.1.114-121.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orberg P. K., Sandine W. E. Common occurrence of plasmid DNA and vancomycin resistance in Leuconostoc spp. Appl Environ Microbiol. 1984 Dec;48(6):1129–1133. doi: 10.1128/aem.48.6.1129-1133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K., Good R. F. A bidirectional rho-independent transcription terminator between the E. coli tonB gene and an opposing gene. Cell. 1985 Jun;41(2):577–585. doi: 10.1016/s0092-8674(85)80030-1. [DOI] [PubMed] [Google Scholar]
- Roepstorff P., Nielsen P. F., Kamensky I., Craig A. G., Self R. Cf plasma desorption mass spectrometry of a polycyclic peptide antibiotic, nisin. Biomed Environ Mass Spectrom. 1988 Mar 15;15(6):305–310. doi: 10.1002/bms.1200150602. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- van Belkum M. J., Hayema B. J., Jeeninga R. E., Kok J., Venema G. Organization and nucleotide sequences of two lactococcal bacteriocin operons. Appl Environ Microbiol. 1991 Feb;57(2):492–498. doi: 10.1128/aem.57.2.492-498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vossen J. M., van der Lelie D., Venema G. Isolation and characterization of Streptococcus cremoris Wg2-specific promoters. Appl Environ Microbiol. 1987 Oct;53(10):2452–2457. doi: 10.1128/aem.53.10.2452-2457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]