Abstract
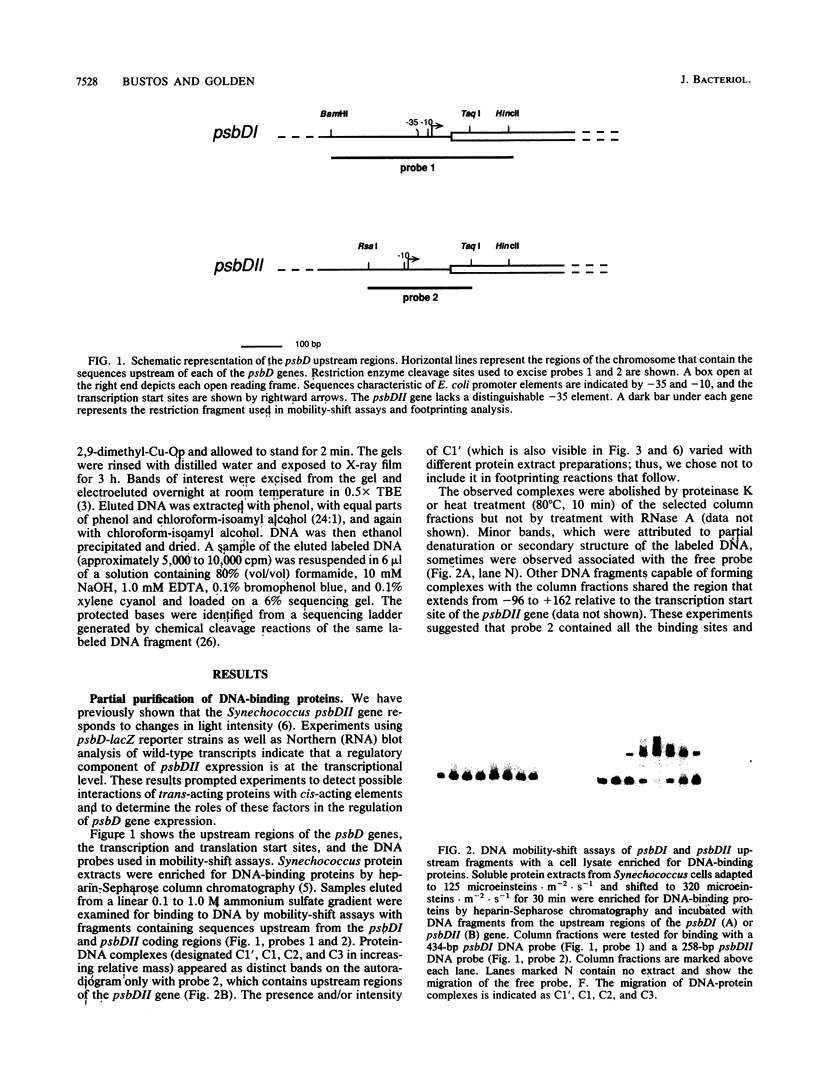
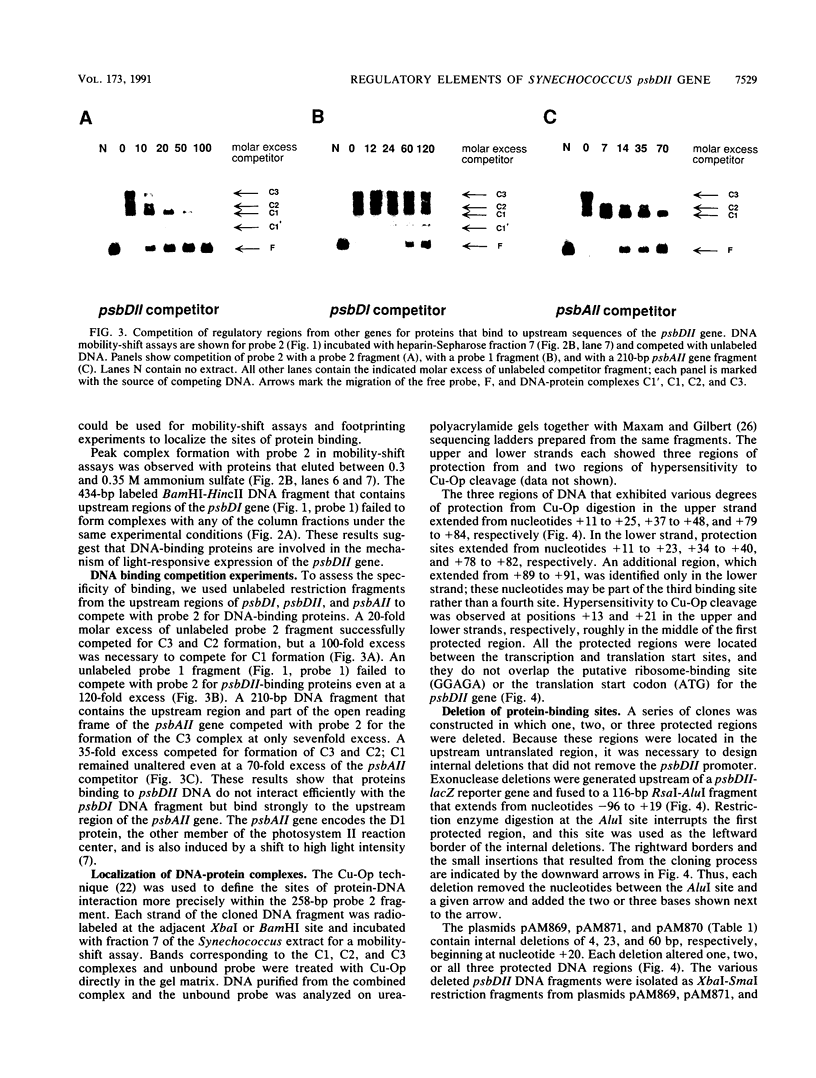
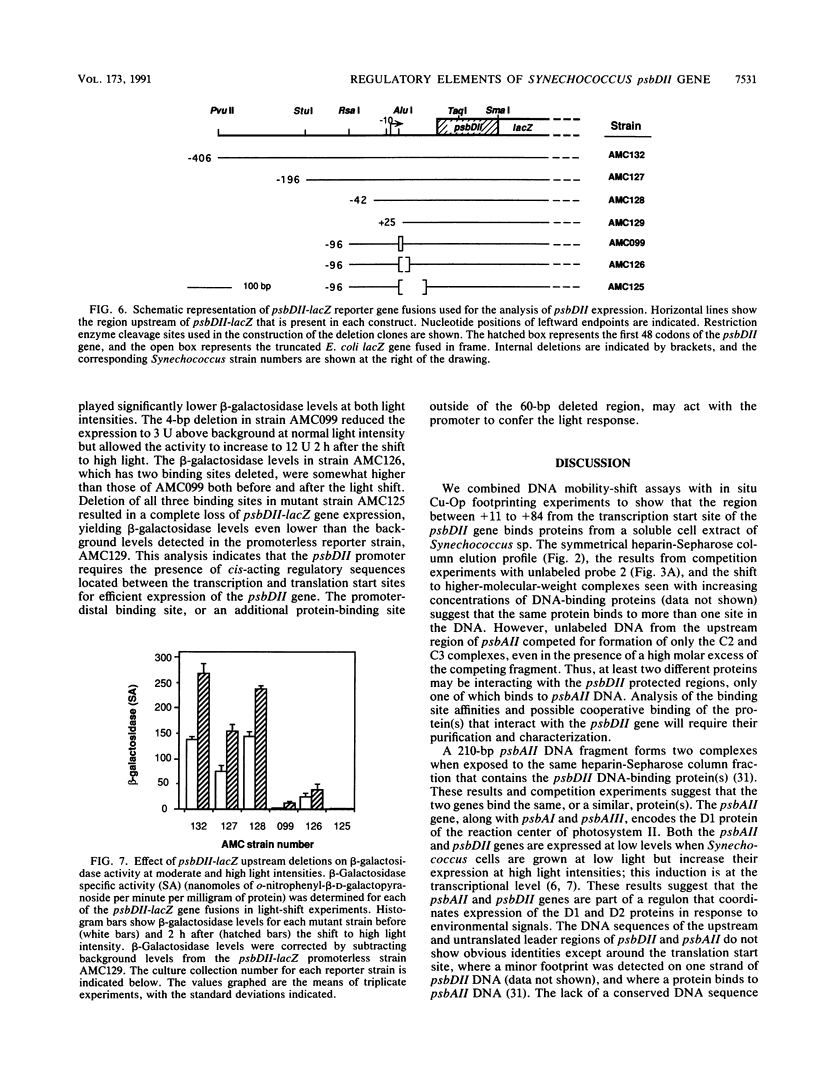
The psbDI and psbDII genes in Synechococcus sp. strain PCC 7942 encode the D2 polypeptide, an essential component of the photosystem II reaction center. Previous studies have demonstrated that transcripts from psbDII, but not psbDI, increase in response to high light intensity. Soluble proteins from Synechococcus cells shifted to high light were found to have affinity for DNA sequences upstream from the psbDII coding region. DNA mobility-shift and copper-phenanthroline footprinting assays of a 258-bp fragment revealed three distinct DNA-protein complexes that mapped to the untranslated leader region between +11 and +84. Deletion of the upstream flanking region to -42 had no effect on the expression of a psbDII-lacZ reporter gene or its induction by light, whereas a promoterless construct supported only minimal background levels of beta-galactosidase. A 4-bp deletion within the first protected region of the footprint decreased the beta-galactosidase activity to approximately 2% of that of the undeleted control, but gene expression remained responsive to light. Deletion of the three protected regions completely abolished both gene expression and light induction. These results suggest that the psbDII gene requires elements within the untranslated leader region for efficient gene expression, one of which may be involved in regulation by light.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arkhipova I. R., Ilyin Y. V. Properties of promoter regions of mdg1 Drosophila retrotransposon indicate that it belongs to a specific class of promoters. EMBO J. 1991 May;10(5):1169–1177. doi: 10.1002/j.1460-2075.1991.tb08057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos S. A., Schaefer M. R., Golden S. S. Different and rapid responses of four cyanobacterial psbA transcripts to changes in light intensity. J Bacteriol. 1990 Apr;172(4):1998–2004. doi: 10.1128/jb.172.4.1998-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain C. J., Brusca J. S., Ramasubramanian T. S., Wei T. F., Golden J. W. A sequence-specific DNA-binding factor (VF1) from Anabaena sp. strain PCC 7120 vegetative cells binds to three adjacent sites in the xisA upstream region. J Bacteriol. 1990 Sep;172(9):5044–5051. doi: 10.1128/jb.172.9.5044-5051.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downard J. S., Kim S. H., Kil K. S. Localization of the cis-acting regulatory DNA sequences of the Myxococcus xanthus tps and ops genes. J Bacteriol. 1988 Oct;170(10):4931–4938. doi: 10.1128/jb.170.10.4931-4938.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A. M., Kushner S. R. Transcription of the uvrD gene of Escherichia coli is controlled by the lexA repressor and by attenuation. Nucleic Acids Res. 1983 Dec 20;11(24):8625–8640. doi: 10.1093/nar/11.24.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Kishi F., Miki T., Kagamiyama H., Nakazawa T., Nakazawa A. The nucleotide sequence surrounding the promoter region of colicin E1 gene. Gene. 1981 Nov;15(2-3):119–126. doi: 10.1016/0378-1119(81)90121-9. [DOI] [PubMed] [Google Scholar]
- Fisher R. F., Long S. R. DNA footprint analysis of the transcriptional activator proteins NodD1 and NodD3 on inducible nod gene promoters. J Bacteriol. 1989 Oct;171(10):5492–5502. doi: 10.1128/jb.171.10.5492-5502.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Cho D. S., Nalty M. S. Two functional psbD genes in the cyanobacterium Synechococcus sp. strain PCC 7942. J Bacteriol. 1989 Sep;171(9):4707–4713. doi: 10.1128/jb.171.9.4707-4713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Stearns G. W. Nucleotide sequence and transcript analysis of three photosystem II genes from the cyanobacterium Synechococcus sp. PCC7942. Gene. 1988 Jul 15;67(1):85–96. doi: 10.1016/0378-1119(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Holschuh K., Bottomley W., Whitfeld P. R. Structure of the spinach chloroplast genes for the D2 and 44 kd reaction-centre proteins of photosystem II and for tRNASer (UGA). Nucleic Acids Res. 1984 Dec 11;12(23):8819–8834. doi: 10.1093/nar/12.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil K. S., Brown G. L., Downard J. S. A segment of Myxococcus xanthus ops DNA functions as an upstream activation site for tps gene transcription. J Bacteriol. 1990 Jun;172(6):3081–3088. doi: 10.1128/jb.172.6.3081-3088.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb A., Spassky A., Chapon C., Blazy B., Buc H. On the different binding affinities of CRP at the lac, gal and malT promoter regions. Nucleic Acids Res. 1983 Nov 25;11(22):7833–7852. doi: 10.1093/nar/11.22.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara M. D., Sigman D. S. Footprinting DNA-protein complexes in situ following gel retardation assays using 1,10-phenanthroline-copper ion: Escherichia coli RNA polymerase-lac promoter complexes. Biochemistry. 1987 Nov 17;26(23):7234–7238. doi: 10.1021/bi00397a006. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mizusawa S., Court D., Gottesman S. Transcription of the sulA gene and repression by LexA. J Mol Biol. 1983 Dec 15;171(3):337–343. doi: 10.1016/0022-2836(83)90097-9. [DOI] [PubMed] [Google Scholar]
- Morden C. W., Golden S. S. Sequence analysis and phylogenetic reconstruction of the genes encoding the large and small subunits of ribulose-1,5-bisphosphate carboxylase/oxygenase from the chlorophyll b-containing prokaryote Prochlorothrix hollandica. J Mol Evol. 1991 May;32(5):379–395. doi: 10.1007/BF02101278. [DOI] [PubMed] [Google Scholar]
- Moura-Neto R., Dudov K. P., Perry R. P. An element downstream of the cap site is required for transcription of the gene encoding mouse ribosomal protein L32. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3997–4001. doi: 10.1073/pnas.86.11.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani Y., Horikoshi M., Brenner M., Yamamoto T., Besnard F., Roeder R. G., Freese E. A downstream initiation element required for efficient TATA box binding and in vitro function of TFIID. Nature. 1990 Nov 1;348(6296):86–88. doi: 10.1038/348086a0. [DOI] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfa A. J., Reitzer L. J., Magasanik B. Initiation of transcription at the bacterial glnAp2 promoter by purified E. coli components is facilitated by enhancers. Cell. 1987 Sep 25;50(7):1039–1046. doi: 10.1016/0092-8674(87)90170-x. [DOI] [PubMed] [Google Scholar]
- Pfeifer K., Prezant T., Guarente L. Yeast HAP1 activator binds to two upstream activation sites of different sequence. Cell. 1987 Apr 10;49(1):19–27. doi: 10.1016/0092-8674(87)90751-3. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Schaefer M. R., Golden S. S. Differential expression of members of a cyanobacterial psbA gene family in response to light. J Bacteriol. 1989 Jul;171(7):3973–3981. doi: 10.1128/jb.171.7.3973-3981.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Hedge P. J., te Heesen S., Edelman A., Broome-Smith J. K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41(2-3):337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Storz G., Ames B. N. Identification and molecular analysis of oxyR-regulated promoters important for the bacterial adaptation to oxidative stress. J Mol Biol. 1989 Dec 20;210(4):709–719. doi: 10.1016/0022-2836(89)90104-6. [DOI] [PubMed] [Google Scholar]
- Wek R. C., Hatfield G. W. Transcriptional activation at adjacent operators in the divergent-overlapping ilvY and ilvC promoters of Escherichia coli. J Mol Biol. 1988 Oct 5;203(3):643–663. doi: 10.1016/0022-2836(88)90199-4. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elzen P. J., Maat J., Walters H. H., Veltkamp E., Nijkamp H. J. The nucleotide sequence of the bacteriocin promoters of plasmids Clo DF13 and Co1 E1: role of lexA repressor and cAMP in the regulation of promoter activity. Nucleic Acids Res. 1982 Mar 25;10(6):1913–1928. doi: 10.1093/nar/10.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]