Abstract
This study demonstrates that neutralizing-antibody-producing B cells, CD4+ T cells, and interferons (IFNs) are of key importance in virus control both in adoptive immunotherapy of persistent infection and in the late phase of acute infection with the WE strain of lymphocytic choriomeningitis virus (LCMV). We report the following results. (i) Clearance of LCMV-WE from C57BL/6 carrier mice by adoptive transfer of memory spleen cells requires B cells and CD4+ T cells but not necessarily CD8+ T cells. (ii) At the doses examined, CD8+ T cells contribute to the initial reduction of viral titers but are alone not sufficient to clear the virus because they are exhausted. (iii) In the presence of functional IFN-γ, virus clearance correlates well with the generation of neutralizing antibodies in the treated carrier mice. (iv) In the absence of receptors for IFN-γ, virus clearance is not achieved. (v) Adoptive immunotherapy of mice persistently infected with a distinct virus isolate, LCMV-Armstrong, revealed only low levels of neutralizing antibodies; in this case, CD8+ T cells were needed for virus clearance in addition to B and CD4+ T cells. (vi) After low dose infection of C57BL/6 mice with LCMV-WE, virus is eliminated below detectable levels by CD8+ T cells, but long-term (>2 months) virus control is usually not achieved in the absence of B cells or CD4+ T cells; reappearance of the virus is paralleled either by exhaustion of virus-specific cytotoxic T lymphocytes or lethal immunopathology. These findings are of importance for adoptive immunotherapy strategies against persistent virus infections in humans.
Control of acute viral infections and of virus clearance from chronic virus carriers by adoptive immunotherapy with immune spleen cells has been studied in the mouse model infection with the noncytopathic lymphocytic choriomeningitis virus (LCMV) for more than 30 years (1–12). For elimination of LCMV from virus carriers, it was shown that hyperimmune sera had no effect (10), but antiviral cytotoxic T lymphocytes (CTL) are capable of reducing viral titers; CTL have, therefore, been invoked as the major effector population (12–15). However, several important observations remain unexplained. (i) Why are transferred spleen cells obtained from mice 8–10 days after infection unable to clear the virus, whereas memory spleen cells from mice more than 60 days after infection are efficient (8)? Although two kinds of immune T cells have been postulated to explain this result (12), an untested explanation could be that neutralizing-antibody responses can be demonstrated only at the later time point (16, 17). We have recently shown that specific infection of B cells expressing the neutralizing-antibody receptor leads to CTL-mediated lysis of infected B cells during the acute phase of LMCV infection (18). (ii) How can adoptively transferred CTL alone mediate virus clearance when confrontation with large amounts of antigen after transfer into carrier mice leads to CTL exhaustion? This has been shown for naive and primed CD8+ T cells in mice persistently infected with LCMV-WE or acutely LCMV DOCILE-infected mice (6, 19). (iii) How can CTL-mediated virus clearance be achieved without causing significant immunopathology? This is particularly important for virus clearance from infected neurons because these cells cannot regenerate (14). Therefore, effector mechanisms other than perforin-mediated cytotoxicity such as antibodies or interferon γ (IFN-γ), which is also released by CD8+ T cells, have been suggested to be involved in clearance of a persistent LCMV infection in carrier mice (20). The importance of effector populations other than CD8+ T cells in the control of noncytopathic virus infections has recently also been emphasized after acute infection with LCMV (21). It was shown that the presence of CD4+ T cells and B cells is critical for long-term virus control.
Clearance of virus from persistently infected hosts is of wide medical interest and adoptive immunotherapy of chronic virus carriers has been attempted in cytomegalovirus (22), Epstein–Barr virus (23), hepatitis B virus (24), and HIV infections (25) in humans by using expanded CTL populations. Therefore, this study reevaluated immune mechanisms critically responsible for LCMV clearance from carrier mice and in long-term control of acute LCMV infection.
MATERIALS AND METHODS
Mice and Viruses.
Inbred C57BL/6 and wild-type 129 (wt129) mice were purchased from the Institut für Versuchstierkunde (University of Zürich, Zürich). Breeding pairs of IFN-α/β or IFN-γ receptor-deficient mice (26, 27), major histocompatibility complex (MHC) class II −/− (28), CD4 −/− (29), and immunoglobulin μ chain gene-deficient mice (30) were originally obtained from the referenced sources and bred locally. Congenital LCMV-WE-carrier mice were offsprings of C57BL/6 or G129 or A129 mice that were injected less than 24 h after birth with 106 plaque-forming units (pfu) of LCMV-WE; alternatively, immunosuppressed females were injected with LCMV-Armstrong (ARM), and infected offsprings were used as carriers. LCMV-WE was obtained from Fritz Lehmann-Grube (Heinrich-Pette Institut für Experimentelle Virologie, Hamburg, Germany). LCMV-ARM CA 1371 was provided by M. Buchmeier (The Scripps Research Institute, La Jolla, CA). Virus titers were quantified using a focus forming assay on MC57G cells (31). Titers are expressed as plaque-forming units per gram of organ or per milliliter of blood.
Depletion of Cell Subsets and Cell Transfer.
For in vitro depletion of CD4+ and CD8+ T cells, spleen cells were incubated with mAb M13 (anti-CD8) and mAb 172.4 (anti-CD4) for 30 min at 4°C, followed by the addition of rabbit complement for 90 min at 37°C. In addition, mAbs coated with microbeads (GK1.5 anti-mouse L3T4, 53–6.7 anti-mouse Ly-2, and RA3–6B2 anti-mouse Ly-5; Milteny Biotec, Bergisch-Gladbach, Germany) were used for positive selection or depletion of lymphocyte subsets by magnetic cell sorting (MACS). Efficiency of purification was controlled by fluorescence activated cell sorter analysis with use of fluorescein isothiocyanate-labeled antibodies (PharMingen). Purity was >95% for all populations used except for MACS-purified CD8+ T cells, for which purity was 80–85%. The cell numbers transferred correspond to the number of viable cells as counted immediately before i.v. injection.
Neutralization and Cytotoxicity Assays.
LCMV-specific neutralizing activity of mouse sera was measured in a focus reduction assay (31). Cytotoxic activity of spleen cells was assessed after 5 days of restimulation in vitro on thioglycolate-induced (1 ml i.p. day −6), LCMV-infected (200 pfu i.p. day −4) peritoneal macrophages against LCMV-WE-infected MC57G target cells in a standard 51Cr-release assay (32).
RESULTS
Clearance of Virus from LCMV-WE-Carrier Mice by Adoptive Transfer of Memory Spleen Cells Requires B and CD4+ T Cells but Not CD8+ T Cells.
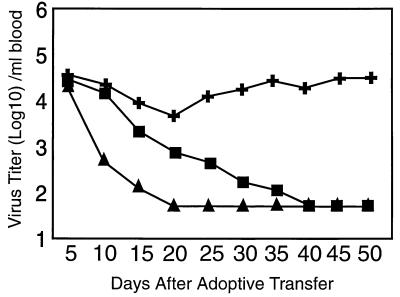
To establish quantitative and kinetic requirements for effective virus control, we adoptively transfused LCMV-WE-infected C57BL/6 carrier mice with 107 or 108 spleen cells from syngeneic mice, which had been infected with 200 pfu of LCMV-WE at least 60 days previously (d60 immune spleen cells). Adoptive transfer of 108 memory spleen cells led to clearance of virus from blood and various organs of recipient carrier mice (Fig. 1 and Table 1, experiment 1) within 10–20 days, whereas 107 spleen cells were not effective (data not shown). Virus clearance from the kidneys was not achieved before day 60, confirming earlier results (8, 13). As had been described in earlier studies, in less than 10% of the mice treated with 108 cells, virus decreased only transiently and persisted (13).
Figure 1.
Delayed virus clearance after adoptive transfer of d60 immune spleen cells depleted of CD8+ T cells. LCMV-WE-immune (200 pfu i.v. >60 days previously) spleen cells were depleted of CD4+ (✚) or CD8+ T cells (▪); 108 cells were adoptively transferred into LCMV-WE-carrier mice. Virus clearance in the blood was compared with LCMV-WE-carrier mice receiving 108 untreated d60 immune spleen cells (▴). Titers are expressed as the mean of 7–9 mice; SEM was <0,4 log10.
Table 1.
B cells and CD4+ T cells but not CD8+ T cells are needed for successful adoptive immunotherapy of LCMV-WE carrier mice
| Exp. | Transferred lymphocytes | Days after transfer | Virus titer
|
|||
|---|---|---|---|---|---|---|
| Blood | Brain | Spleen | Kidney | |||
| 1 | d60 immune cells | 20 | <1.8 | <1.9 | <2.1 | 4.8 |
| d8 immune cells | 20 | 2.6 ± 0.6 | 4.5 ± 0.6 | 4.4 ± 0.3 | 5.8 ± 0.2 | |
| None | 6.9 ± 0.3 | 6.2 ± 0.2 | 6.0 ± 0.3 | 7.1 ± 0.4 | ||
| d60 immune cells | ||||||
| 2 | anti-CD8 + C′ treated | 90 | <1.8 | <1.8 | <2.2 | 3.4 ± 0.3 |
| anti-CD4 + C′ treated | 90 | 5.7 ± 0.1 | 4.6 ± 0.3 | 5.6 ± 0.3 | 6.6 ± 0.2 | |
| anti-B cell + C′ treated | 90 | 4.2 ± 0.3 | 5.1 ± 0.4 | 5.3 ± 0.4 | 6.3 ± 0.3 | |
| 3 | CD8 depleted | 40 | <1.7 | <2.0 | <2.0 | 4.4 ± 0.2 |
| CD4 depleted | 40 | 2.3 ± 0.6 | 2.5 ±0.3 | 3.5 ± 0.7 | 5.3 ± 0.2 | |
| 4 | CD4+ plus CD8+ cells | 40 | 4.6 ± 0.1 | 5.5 ± 0.1 | 6.5 ± 0.1 | 6.8 ± 0.1 |
| CD8+ plus B cells | 40 | 3.8 ± 0.4 | 4.7 ± 0.1 | 5.7 ± 1.1 | 5.9 ± 0.1 | |
| CD4+ plus B cells | 40 | <1.7 | <2.0 | <2.1 | 4.5 ± 0.3 | |
C57BL/6 mice were infected i.v. with 200 pfu of LCMV-WE. Spleen cells (108) of these mice were taken either 8 days (d8 immune cells) or 60 days after infection (d60 immune cells), and cells were adoptively transferred to C57BL/6 LCMV-WE-infected carrier mice.
Virus titers are expressed as plaque-forming units per gram of organ or per milliliter of blood (log10 + SEM) and are means ± sem of four mice. Experiments were repeated at least twice with similar results. In experiment (Exp.) 2, either CD4+ or CD8+ T cells or B cells were depleted with mAbs and complement. In experiment 3, CD4+ or CD8+ T cells were depleted with the use of magnetic beads. Cell depletion efficacy was >95%. In experiment 4, CD4+ T cells, CD8+ T cells, and B cells were purified by magnetic beads. Then, 5 × 106 CD4+ T cells, 5 × 106 CD8+ T cells, and 1 × 107 B cells (previously determined as minimally necessary numbers of cells) were injected in various combinations into LCMV-WE carriers. The purity of the enriched spleen cell populations was >95% for B and CD4+ T cells and 80–85% for CD8+ T cells.
To analyze which lymphocyte populations were responsible for virus clearance, specific cell subsets were depleted from d60 immune spleens by using either mAbs and complement or cell separation with magnetic beads (MACS). Adoptive transfer of 108 d60 immune spleen cells depleted of either CD4+ T cells or B cells did not lead to virus clearance from LCMV-WE-carrier mice (Table 1, experiments 2 and 3). Surprisingly and in contrast to results obtained with LCMV-ARM (see below), LCMV-WE-carrier mice were able to clear the persistent infection if treated with 108 d60 immune spleen cells that were depleted of CD8+ T cells (Table 1, experiments 2 and 3); however, clearance was slower and was not achieved before day 40 in the blood (Fig. 1). These results were confirmed by a series of adoptive transfer experiments using MACS-purified d60 immune cells. To limit the number of donor spleens, minimal cell doses needed to achieve virus clearance were used in these experiments. Of the various combinations of positively selected 107 B cells, 5 × 106 CD4+ T cells, or 5 × 106 CD8+ T cells, only a combination of CD4+ T cells plus B cells succeeded in clearing LCMV-WE-carrier mice, whereas all other combinations had no long-term effect on virus titers (Table 1, experiment 4).
Neutralizing-Antibody-Producing B Cells Are the Limiting Cell Population for Clearance of LCMV-WE from Carrier Mice by Adoptive Immunotherapy.
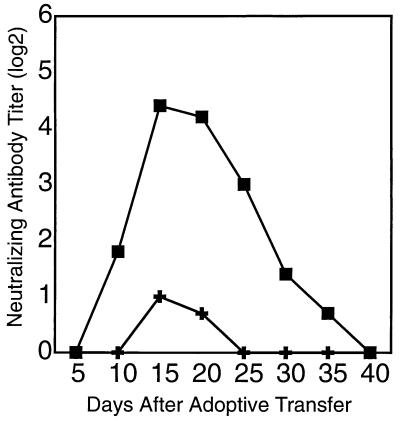
Previous experiments had shown that LCMV-specific neutralizing antibodies cannot be detected before ≈60 days after infection with 200 pfu of LCMV-WE (16, 17). An important role for neutralizing-antibody-producing B cells in virus clearance from carrier mice would imply that only donor spleen cells from mice infected more than 60 days earlier should be effective. As also shown in earlier studies using different virus isolates (8, 12), adoptive transfer of spleen cells from mice infected with LCMV-WE 8 days previously (d8 immune spleen cells) failed to clear the persistent infection (Table 1, experiment 1). Also a combination of 1 × 107 d8 immune B cells and 5 × 106 d60 memory CD4+ cells was ineffective (Table 2). In contrast, adoptive transfer of 1 × 107 d60 immune B cells in combination with 5 × 106 d8 immune CD4+ T cells mediated virus clearance from LCMV-WE-carrier mice within 40 days (Table 2). Thus, although primed CD4+ T cells are necessary, the limiting cell population for clearance of LCMV-WE from carrier mice appear to be neutralizing-antibody-producing B cells. To test whether neutralizing antibodies were detectable in carrier mice undergoing successful adoptive immunotherapy, we analyzed serum of the recipient mice at different time points after adoptive transfer of 108 d60 immune spleen cells using neutralization assays. Fig. 2 shows that 9–12 days after cell transfer neutralizing antibody titers could be detected. The titers increased until days 15–20 and then decreased until about day 30, when virus had been cleared and, surprisingly, no more neutralizing antibody activity could be detected.
Table 2.
Successful virus clearance from LCMV-WE carrier mice requires d60 immune B cells
| Combination of transferred lymphocytes* (CD4/CD8/B) | Virus titer†
|
NAb titers‡ | |||
|---|---|---|---|---|---|
| Blood | Brain | Spleen | Kidney | ||
| Unseparated spleen cells (d60) | <1.7 | <2.0 | <2.0 | 4.7 ±0.5 | 3/3 |
| d8/d60/d60 | <1.7 | <1.8 | <2.0 | 3.8 ± 0.4 | 3/3 |
| d8/—/d60 | <1.7 | <2.0 | <2.0 | 4.6 ± 0.3 | 3/3 |
| d60/d8/d8 | 3.9 ± 0.3 | 4.8 ± 0.5 | 4.5 ± 0.5 | 5.5 ± 0.6 | 0/3 |
| d60/—/d8 | 4.2 ± 0.5 | 4.7 ± 0.2 | 4.2 ± 1.1 | 5.7 ± 0.4 | 0/3 |
Spleen cells from mice infected i.v. with 200 pfu of LCMV-WE either 8 or 60 days previously were depleted of CD4+ or CD8+ T cells or B cells or positively selected for CD4+ or CD8+ T cells or B cells using magnetic beads. Then, 5 × 106 CD4+ T cells, 5 × 106 CD8+ T cells, and 1 × 107 B cells from d8 or d60 immune spleens were injected in various combinations (cell order shown is CD4/CD8/B cells) into LCMV-WE-carrier mice according to the experimental setups listed in the table.
Virus titers are expressed as plaque-forming units per milliliter of blood or gram of organ (log10) on day 40 after cell transfer and are means ± SEM of three mice. The experiments were repeated at least three times with similar results.
Neutralizing antibody (NAb) titers were monitored 15–30 days after adoptive transfer, and numbers of mice displaying titers >2 log2 per total number of mice are given in the table.
Figure 2.
Neutralizing antibodies are more efficiently induced in LCMV-carrier mice by adoptive transfer of LCMV-WE-primed than LCMV-ARM-primed memory spleen cells. LCMV-WE-immune (▪) or LCMV-ARM-immune (✚) spleen cells (108) were adoptively transferred into the corresponding C57BL/6 LCMV-carrier mice. Sera were analyzed for neutralizing antibody activity (data are the mean of 3–5 mice; SEM < 1). The experiment was repeated four times with similar results.
CD8+ T Cells Are Needed in Addition to B and CD4+ T Cells for Virus Clearance from Carrier Mice, if Adoptive Immunotherapy Does Not Induce Significant Titers of Neutralizing Antibodies.
The results presented above are in contrast to previous studies using the ARM and Traub isolates of LCMV; these studies characterized CTL as the limiting cell population for virus clearance (12, 13, 15, 33). Therefore, a series of experiments using LCMV-ARM-carrier mice was added. Table 3 shows that adoptive transfer of 108 d60 LCMV-ARM-immune spleen cells caused virus clearance, albeit with a significantly slower kinetics than that observed for LCMV-WE (60 versus 10–20 days). In contrast to LCMV-WE, clearance of LCMV-ARM from carrier mice required B and CD4+ T cells as well as CD8+ T cells (Table 3); interestingly, the sera of LCMV-ARM-carrier mice undergoing immunotherapy contained only low titers of neutralizing antibodies (Fig. 2). This result suggests that when low titers of neutralizing antibodies are induced, CTL were necessary in addition to B and CD4+ T cells for virus clearance. These findings were further corroborated by experiments that were based on the cross-reaction of LCMV-specific CTL and neutralizing antibodies between the ARM and WE isolates. LCMV-ARM-carrier mice adoptively transfused with d60 LCMV-WE-immune spleen cells cleared virus by around 20 days after transfer (Table 3), and the kinetics of neutralizing antibody titers was similar to that obtained in LCMV-WE-carrier mice. CD8+ T cell depletion abrogated the capacity to clear. In contrast, LCMV-WE-carrier mice that received 108 LCMV-ARM-primed memory spleen cells did not clear the persistent virus infection (Table 3), and no neutralizing antibodies could be measured.
Table 3.
Successful adoptive immunotherapy of LCMV-ARM-carrier mice requires LCMV-ARM-primed B cells and CD4+ T cells as well as CD8+ T cells
| Exp. | Transferred lymphocytes | Virus titer*
|
|||
|---|---|---|---|---|---|
| Blood | Brain | Spleen | Kidney | ||
| 1 | d60 ARM immune cells | <1.7 | <1.8 | <2.1 | 4.2 ± 0.2 |
| d8 ARM immune cells | 3.7 ± 0.4 | 4.6 ± 0.4 | 5.2 ± 0.3 | 5.8 ± 0.4 | |
| 2 | d60 ARM immune cells | ||||
| CD8+ T cell depleted | 4.6 ± 0.9 | 4.4 ± 0.5 | 5.7 ± 0.4 | 6.0 ± 0.5 | |
| CD4+ T cell depleted | 3.4 ± 0.3 | 4.8 ± 0.2 | 5.6 ± 0.6 | 6.0 ± 0.2 | |
| B cell depleted | 4.2 ± 0.3 | 6.2 ± 0.5 | 5.7 ± 0.4 | 6.4 ± 0.2 | |
| 3 | WE-immune cells into ARM-carrier mice | <1.7 | <1.7 | <2.1 | 3.3 |
| ARM-immune cells into WE-carrier mice | 4.4 ± 0.4 | 4.4 ± 0.3 | 4.6 ± 0.6 | 6.2 ± 0.5 | |
In experiment (Exp.) 1, C57BL/6 mice were infected i.v. with 200 pfu of LCMV-ARM. Spleen cells of these mice were taken either 8 days (d8 ARM-immune cells) or 60 days (d60 ARM-immune cells) after infection, and 108 cells were adoptively transferred into C57BL/6 mice persistently infected with LCMV-ARM. In experiment 2, specific lymphocyte subpopulations were depleted by magnetic cell sorting before transfer. In experiment 3, 108 d60 LCMV-ARM-immune or d60 LCMV-WE-immune spleen cells were adoptively transferred into C57BL/6 mice persistently infected with one of the two LCMV isolates. At 100 days after cell transfer, the carrier mice were killed and virus was quantified.
Virus titers are expressed as plaque-forming units per milliliter of blood or gram of organ (log10) on day 100 after cell transfer and are means ± SEM of three to five mice. Experiments were repeated twice with similar results.
IFNs Are Important for Clearance of a Persistent LCMV Infection in Carrier Mice.
IFNs have previously been implicated in virus clearance from carrier mice (20). To further define the role of IFNs, LCMV-WE-carrier mice that lacked the receptor for either IFN-γ (G129 mice; ref. 27) or IFN-α/β (A129 mice; ref. 28) were analyzed in adoptive transfer experiments. Because these mice have a 129 strain background, wt129 LCMV-carrier mice were used as controls. Adoptive transfer of 108 >d60 immune wt129 spleen cells led to transient clearance of virus from both A129 and G129 LCMV-carrier mice (Table 4). However, clearance was not complete. In the absence of the IFN-α/β receptor, virus persisted at very low levels in several organs as late as 60 days after transfer. In IFN-γ receptor-deficient carrier mice, virus titers were overall only slightly reduced. Both of the mutant carrier mice displayed high titers of neutralizing antibodies (Table 4).
Table 4.
Both IFN-α/β and IFN-γ are necessary for virus clearance in addition to neutralizing antibodies
| Transferred lymphocytes* | LCMV-WE carrier | Neutralizing Ab titer (log2)
|
Virus titer† per ml blood or gram organ (log10) on d60 after cell transfer
|
||||
|---|---|---|---|---|---|---|---|
| d10 | d20 | Blood | Brain | Spleen | Kidney | ||
| wt129 | wt129 | 8.0 | 7.5 | <1.7 | <1.6 | <2.0 | 3.2 |
| wt129 | A129 | 4.3 | 6.0 | 2.2 | <1.6 | 2.6 | 5.2 |
| wt129 | G129 | 5.0 | 3.8 | 4.8 | 4.8 | 4.0 | 5.6 |
wt129 mice were infected i.v. with 200 pfu of LCMV-WE. Spleen cells of these mice were taken 60 days after infection, and 108 cells were adoptively transferred into either wild-type (wt129), IFN-α/β receptor-deficient (A129) or IFN-γ receptor-deficient (G129) LCMV-WE-carrier mice. On days 10 and 20 after transfer, the blood of the treated carrier mice was monitored for infectious virus and for the appearance of neutralizing antibodies.
Virus titers are expressed as plaque-forming units per milliliter of blood or gram of organ (log10) on day 60 after cell transfer and are means of two to four mice; SEM. < 0,4 log10. The experiment was repeated twice with similar results.
B Cells and CD4+ T Cells Are Also Needed for Long-Term Virus Control After Acute Low Dose Infection with LCMV-WE.
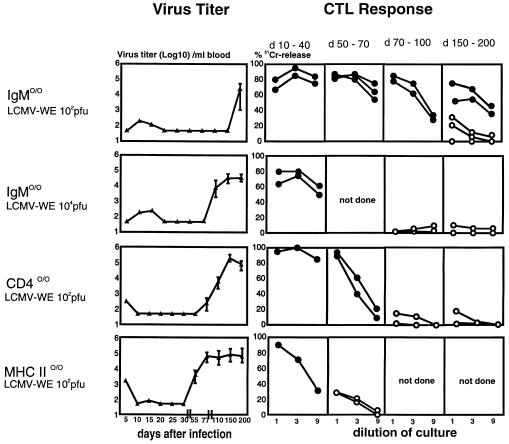
Is there a role for B cells and CD4+ T cells in control of acute LCMV-WE infection? After infection of CD4+ T cell-, MHC class II-, or B cell-deficient mice with 200 pfu of LCMV-WE, virus titers in the blood (Fig. 3) and in the spleen, lung, brain, and liver (data not shown) dropped below detectable levels in all three mutant mouse strains examined by day 20, confirming earlier results (30, 34–37). However, virus could again be detected both in the blood and in solid organs around day 50 after infection of MHC class II-deficient mice, around day 70 in CD4-deficient mice, and as late as day 200 in B cell-deficient mice (Fig. 3). The time of reappearance of the virus was dose-dependent, as demonstrated here for B cell-deficient mice infected with 104 versus 102 pfu of LCMV-WE. Among the latter, 2 of 21 mice remained free of virus throughout the observation period of 230 days.
Figure 3.
Long-term virus control after LCMV infection depends on the presence of B cells and CD4+ T cells. IgM −/− and CD4- and MHC class II-deficient mice were infected with low (102 pfu) or intermediate (104 pfu) doses of LCMV-WE, and virus titers were measured in the blood (mean of three to six mice; SEM < 0,5 log10). The experiment was repeated twice with similar results. Within the indicated time intervals, two to three mice per group were killed and spleen cells were tested for LCMV-specific cytotoxicity after 5 days of restimulation with LCMV-infected peritoneal macrophages. Spontaneous 51Cr release was <25% in all assays. Open symbols represent CTL activity from mice in which LCMV reappeared, and solid symbols represent CTL activity from mice in which no virus could be detected.
The fact that all of the mutant mouse strains examined initially generated functional CTL memory responses (Fig. 3) raised the question of why these CTL lost control over the virus. Virus isolated from mice late after infection was readily recognized by LCMV-specific CTL, as evidenced by lysis of infected target cells and in vitro restimulation assays (data not shown), rendering the selection of CTL epitope escape mutants unlikely. Also, virus-specific CTL activity could readily be demonstrated by using in vitro restimulation assays in all three mouse strains during the entire time of effective virus control (Fig. 3). The two virus-free B cell-deficient mice showed measurable CTL responses as late as 200 days after LCMV infection, confirming that, in principle, maintenance of CTL memory is independent of B or CD4+ T cells (34–39). However, in none of the mice in which virus had reappeared could virus-specific CTL be demonstrated (Fig. 3). Seven of 21 B cell-deficient mice died between day 150 and day 200 after infection with 200 pfu of LCMV-WE; the first clinical signs of illness correlated with reappearance of virus in the blood. No obvious histopathological signs for T cell-mediated immunopathology were found in these mice (data not shown).
DISCUSSION
This report documents an important role for neutralizing-antibody-producing B cells and CD4+ T cells for efficient adoptive immunotherapy of persistent LCMV-WE or LCMV-ARM infection and shows a decisive contribution of mainly IFN-γ and to a minor extent of IFN-α/β; in addition, a critical role of neutralizing antibodies is shown in long-term control of acute infection with low doses of LCMV-WE. Because initial virus control by CTL remains apparently incomplete in both of these important model situations, continuously produced neutralizing antibodies are apparently critically needed to control virus spread, most likely from peripheral sites of low-level persistence.
The Role of B and CD4+ T Cells Versus CD8+ T Cells in LCMV Clearance from Carrier Mice.
In the presented experiments, virus clearance by adoptive immunotherapy of LCMV-WE-infected virus carrier mice required B cells and CD4+ T cells but not CD8+ T cells. Earlier experiments with carrier mice infected with the ARM isolate of LCMV (14, 15) had shown that 30 days after adoptive transfer of immune spleen cells around half of the mice treated with B cell- or CD4+ T cell-depleted memory spleen cells had cleared virus from the blood, whereas in mice treated with CD8+ T cell-depleted spleen cells, LCMV could still be detected (14, 15). When we repeated these experiments using a more stringent definition of virus clearance (i.e., the absence of virus from blood and several organs 100 days after cell transfer), we found that all three subpopulations in LCMV-ARM-primed donor spleen cells were necessary to achieve virus clearance. What could explain the differences between clearance of LCMV-ARM and LCMV-WE in carriers? Qualitative and/or quantitative differences in the immune donor cell populations are conceivable; on the other hand, spread and distribution of the two virus isolates in the host could be different. We found that WE-induced memory spleen cells could clear virus from ARM carriers, albeit only in the presence of CD8+ T cells, whereas the inverse combination was not effective. This finding suggests that differences both in the donor cells and in the virus present in the recipient are relevant. More importantly, in the presence of IFN, the induction of neutralizing antibodies was a critical prerequisite for virus clearance in these situations, as in all other situations tested; if neutralizing antibody titers were low, CD8+ T cells were, however, needed in addition to B and CD4+ T cells.
Why Have Neutralizing B Cell Responses Been Missed So Far?
The importance of neutralizing-antibody-producing B cells in virus control and elimination probably has been underestimated in previous studies because these protective antibodies cannot be assessed by ELISA assays. Although it seems reasonable to assume that neutralizing antibodies eliminate virus from the blood, it remains unclear whether additional mechanisms may be involved. It is important to note that neutralizing antibodies, i.e., high doses of hyperimmune serum alone, are not sufficient for virus clearance (10). This either reflects rapid consumption by excess virus or shows that the necessary high antibody concentrations provided by B cells homing to sites of virus persistence cannot be achieved by passive transfer of serum. Alternatively, antibody could be rapidly consumed by binding to an excess of virus. The finding that neutralizing-antibody titers rapidly faded once virus was cleared is surprising. In many virus infections, neutralizing antibodies, once induced, usually persist for a long time if not for life. It is tempting to speculate that during immunotherapy of LCMV carriers, complete clearance of virus may deplete antigen depots below a level needed for the maintenance of immunological memory. Our results can provide an explanation of why only late d60 immune, but not early d8 immune, spleen cells can mediate virus clearance (8); neutralizing antibodies appear relatively late (>60 days) after virus infection (16, 17), probably because early neutralizing-antibody-producing B cells are preferentially infected and lysed by CTL (18).
The Role of IFNs and of CTL in LCMV Clearance from Carrier Mice.
Our results obtained with IFN receptor-deficient mice continue data with IFN-γ −/− mice (21) on the role for mainly IFN-γ and also reveal a minor effect of IFN-α/β action on recipient host cells in virus clearance from carrier mice. In this context, the role of CD4+ T cells in elimination of LCMV from persistently infected mice is probably not only to provide help to B cells but also to secrete IFNs and/or other cytokines. What then is the role of CTL in virus clearance from carrier mice? Our results demonstrate that CD8+ T cells contribute primarily early, i.e., within the first 10–20 days, after adoptive immunotherapy. These findings are compatible with results from several studies that signaled a role for CD8+ T cells (12, 14, 15). The contribution of CD8+ T cells appears transient, however, which is in keeping with the previous finding that LCMV-specific CTL may be exhausted after adoptive transfer into carrier mice (6, 19). In this study, 15–20 days after transfer of spleen cells from memory mice, no LCMV-specific cytotoxicity could be recovered from the spleens of recipient mice by using conventional in vitro restimulation assays (40). It, therefore, seems unlikely that immune CTL contribute significantly to virus clearance after this time period. This interpretation seemingly contradicts earlier experiments, which identified LCMV-specific CTL activity in cured carrier mice derived from the original donor spleen used for adoptive immunotherapy (15, 41). The fact that donor-derived CTL can be retrieved late after virus elimination does not, however, prove that these cells are memory CTL which have contributed to the initial viral clearance from the carrier recipient. Exhaustion of CTL is apparently critically dependent on the quantitative balance between antigen and CTL; therefore, our findings do not exclude that this limitation may be at least partially overcome by a very high number of specific CD8+ T cells such as may be used in therapeutic protocols with in vitro expanded T cells. However, this approach implies the danger of either selecting virus escape variants (42) or inducing significant immunopathology or even death (6, 43, 44).
Role of Neutralizing Antibodies in Long-Term Control of LCMV After Low Dose Acute Infection.
While our studies on the role of B cells and CD4+ T cells in long-term control of LCMV infection were in progress, a study on the inability of IgM −/− and MHC class II −/− mice to control acute infection with 1,000 LD50 of LCMV Traub was published (21). The present study confirms and extends these findings for infection of C57BL/6 mice with LCMV-WE. Using low doses of LCMV-WE, we could not demonstrate virus after the acute phase of the infection in any organ except for some kidneys for a period as long as 200 days in the case of IgM −/− mice. This result suggests that virus control in this time period is achieved to a near complete extent; conventional methods are apparently not sensitive enough to detect persisting virus. Because CTL memory could be demonstrated in virus-free IgM −/− mice as late as 200 days after priming, the maintenance of T cell memory appears to be independent of B or CD4+ T cells, as shown previously (34–37). It therefore seems likely that virus, which has not mutated the relevant CTL epitopes, reappears in the presence of functional CTL memory. Of interest, 7 of 21 IgM −/− mice died during the period of 150–200 days after infection. Overall this may suggest an unfortunate balance between two extreme situations; either CTL memory controls virus efficiently at very low levels or virus reappearing spreads too rapidly and very widely so as to cause exhaustion of the virus-specific CTL, allowing the mice to survive as carriers, or there is an intermediate distribution kinetics of the virus so that CTL are not completely exhausted but may eventually cause lethal immunopathology.
Conclusion.
Taken together, the various data on long-term virus control after acute infection with a low dose of LCMV and those on virus clearance from carrier mice correlate well. Initial virus control mediated by CTL is incomplete; LCMV persists at low levels in peripheral sites, from which it may periodically spread throughout the circulation. The obvious implication would be that virus persists after acute LCMV infection, thereby providing the basis for restimulation of protective, i.e., activated CTL memory (45, 46). The following principles emerge for effective immunotherapy of LCMV infection. Neutralizing antibody-producing B cells and CD4+ T cells are the limiting cell populations for effective and long-lasting virus clearance. CD8+ T cells significantly contribute to virus clearance in the early phase, but the relative requirements for a contribution of CD8+ T cells depends on the kinetics and magnitude of the antibody response, which differ in immunotherapy of mice infected with different LCMV isolates. The CTL-independent clearance mechanisms such as IFNs and neutralizing antibodies could explain why clearance of virus from carrier mice can occur in the absence of any demonstrable immunopathology, e.g., in the brain, where CTL-mediated lysis could cause irreversible damage (14). The presented data suggest that long-term induction of neutralizing antibody responses might be critical for successful virus elimination in adoptive immunotherapy of human virus infections with viruses that have the tendency to persist, such as cytomegalovirus, Epstein–Barr virus, hepatitis B virus, or HIV.
Acknowledgments
We thank Paul Klenermann and Anne Rensing-Ehl for helpful discussions and critical reading of the manuscript and Alana Althage for excellent technical assistance. This work was supported by Swiss National Science Foundation Grant 31–32195.91, the Deutsche Forschungsgemeinschaft (S.E.), and the Kanton Zürich.
ABBREVIATIONS
- LCMV
lymphocytic choriomeningitis virus
- CTL
cytotoxic T lymphocyte(s)
- pfu
plaque-forming units
- IFN
interferon
- wt
wild type
- d60 cells
cells from mice infected with LCMV at least 60 days before testing
- MACS
magnetic cell sorting
- MHC
major histocompatibility complex
References
- 1.Cole G A, Nathanson N, Prendergast R A. Nature (London) 1972;238:335–337. doi: 10.1038/238335a0. [DOI] [PubMed] [Google Scholar]
- 2.Doherty P C, Zinkernagel R M. Transplant Rev. 1974;19:89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 3.Zinkernagel R M, Welsh R M. J Immunol. 1976;117:1495–1502. [PubMed] [Google Scholar]
- 4.Hotchin J. Cold Spring Harbor Symp Quant Biol. 1962;27:479–501. doi: 10.1101/sqb.1962.027.001.046. [DOI] [PubMed] [Google Scholar]
- 5.Buchmeier M J, Welsh R M, Dutko F J, Oldstone M B A. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 6.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Nature (London) 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 7.Volkert M. Acta Pathol Microbiol Scand. 1962;56:305–310. [PubMed] [Google Scholar]
- 8.Volkert M. Acta Pathol Microbiol Scand. 1963;57:465–487. [PubMed] [Google Scholar]
- 9.Volkert M, Larsen J H, Pfau C J. Acta Pathol Microbiol Scand. 1964;61:268–282. doi: 10.1111/apm.1964.61.2.268. [DOI] [PubMed] [Google Scholar]
- 10.Volkert M, Larsen J H. Acta Pathol Microbiol Scand. 1965;63:172–180. doi: 10.1111/apm.1965.63.2.172. [DOI] [PubMed] [Google Scholar]
- 11.Volkert M, Larsen J H. Acta Pathol Microbiol Scand. 1965;63:161–171. doi: 10.1111/apm.1965.63.2.161. [DOI] [PubMed] [Google Scholar]
- 12.Volkert M, Marker O, Bro Jorgensen K. J Exp Med. 1974;139:1329–1343. doi: 10.1084/jem.139.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed R, Jamieson B D, Porter D D. J Virol. 1987;61:3920–3929. doi: 10.1128/jvi.61.12.3920-3929.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oldstone M B, Blount P, Southern P J, Lampert P W. Nature (London) 1986;321:239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- 15.Jamieson B D, Butler L D, Ahmed R. J Virol. 1987;61:3930–3937. doi: 10.1128/jvi.61.12.3930-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehmann-Grube F. Virol Monogr. 1971;10:1–37. [Google Scholar]
- 17.Battegay M, Moskophidis D, Waldner H, Bründler M-A, Fung-Leung W-P, Mak T W, Hengartner H, Zinkernagel R M. J Immunol. 1993;151:5408–5415. [PubMed] [Google Scholar]
- 18.Planz O, Seiler P, Hengartner H, Zinkernagel R M. Nature (London) 1996;382:726–729. doi: 10.1038/382726a0. [DOI] [PubMed] [Google Scholar]
- 19.Moskophidis D, Laine E, Zinkernagel R M. Eur J Immunol. 1993;23:3306–3311. doi: 10.1002/eji.1830231237. [DOI] [PubMed] [Google Scholar]
- 20.Tishon A, Lewicki H, Rall G, von Herrath M, Oldstone M B. Virology. 1995;212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 21.Thomsen A R, Johansen J, Marker O, Christensen J P. J Immunol. 1996;157:3074–3080. [PubMed] [Google Scholar]
- 22.Riddell S R, Watanabe K S, Goodrich J M, Li C R, Agha M E, Greenberg P D. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 23.Papadopoulos E B, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi M H, Castro Malaspina H, Childs B H, Gillio A P, Small T N, Young J W, Kernan N A, O‘Reilly R J. N Engl J Med. 1994;330:1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 24.Kohler P F, Trembath J, Merrill D A, Singleton J W, Dubois R S. Clin Immunol Immunopathol. 1974;2:465–471. [Google Scholar]
- 25.Ho M, Armstrong J, McMahon D, Pazin G, Huang X L, Rinaldo C, Whiteside T, Tripoli C, Levine G, Moody D, Oraoura T, Elder E, Gupta P, Tauxe N, Torpey D, Heberman R. Blood. 1993;81:2093–2101. [PubMed] [Google Scholar]
- 26.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 27.Müller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 28.Gosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Cell. 1991;66:1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 29.Rahemtulla A, Fung-Leung W-P, Schilham M W, Kündig T M, Sambhara S R, Narendran A, Arabian A, Wakeham A, Paige C J, Zinkernagel R M, Miller R G, Mak T W. Nature (London) 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura D, Roes J, Kuhn R, Rajewsky K. Nature (London) 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 31.Battegay M, Cooper S, Althage A, Baenziger J, Hengartner H, Zinkernagel R M. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 32.Zinkernagel R M, Haenseler E, Leist T, Cerny A, Hengartner H, Althage A. J Exp Med. 1986;164:1075–1092. doi: 10.1084/jem.164.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oldstone M B A. Curr Top Microbiol Immunol. 1987;134:211–229. doi: 10.1007/978-3-642-71726-0_9. [DOI] [PubMed] [Google Scholar]
- 34.Bründler M-A, Aichele P, Bachmann M, Kitamura D, Rajewsky K, Zinkernagel R M. Eur J Immunol. 1996;26:2257–2262. doi: 10.1002/eji.1830260943. [DOI] [PubMed] [Google Scholar]
- 35.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak T W, Zinkernagel R M. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battegay M, Bachmann M F, Burkhart C, Viville S, Benoist C, Mathis D, Hengartner H, Zinkernagel R M. Cell Immunol. 1996;167:115–121. doi: 10.1006/cimm.1996.0014. [DOI] [PubMed] [Google Scholar]
- 37.Matloubian M, Concepcion R J, Ahmed R. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asano M S, Ahmed R. J Exp Med. 1996;183:2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Rosa F, Matzinger P. J Exp Med. 1996;183:2153–2163. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castelmur I, DiPaolo C, Bachmann M F, Hengartner H, Zinkernagel R M, Kündig T M. Cell Immunol. 1993;151:460–466. doi: 10.1006/cimm.1993.1254. [DOI] [PubMed] [Google Scholar]
- 41.Jamieson B D, Ahmed R. J Exp Med. 1989;169:1993–2005. doi: 10.1084/jem.169.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pircher H, Moskophidis D, Rohrer U, Bürki K, Hengartner H, Zinkernagel R M. Nature (London) 1990;346:629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- 43.Johnson E D, Cole G A. J Exp Med. 1975;141:866–881. [PMC free article] [PubMed] [Google Scholar]
- 44.Byrne J A, Oldstone M B. J Immunol. 1986;136:698–704. [PubMed] [Google Scholar]
- 45.Kündig T M, Bachmann M F, Ohashi P S, Pircher H, Hengartner H, Zinkernagel R M. Immunol Rev. 1996;150:63–97. doi: 10.1111/j.1600-065x.1996.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 46.Zinkernagel R M, Bachmann M F, Kündig T M, Oehen S, Pircher H, Hengartner H. Annu Rev Immunol. 1996;14:333–372. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]