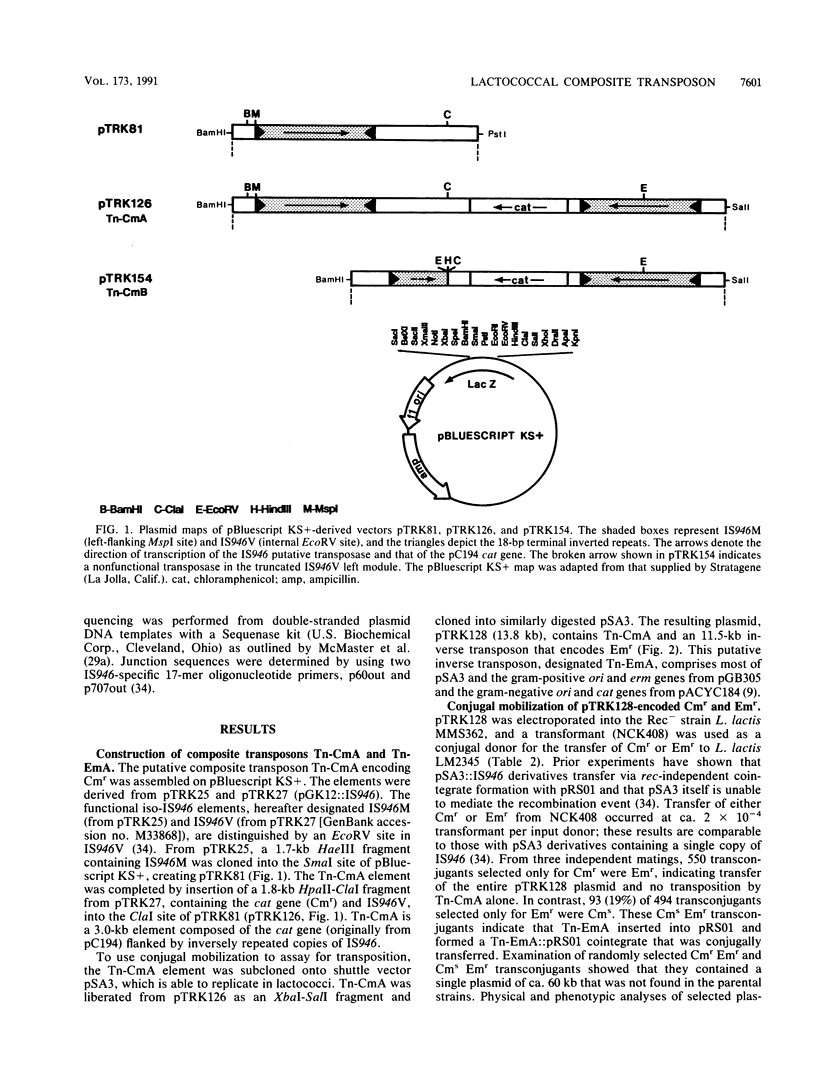
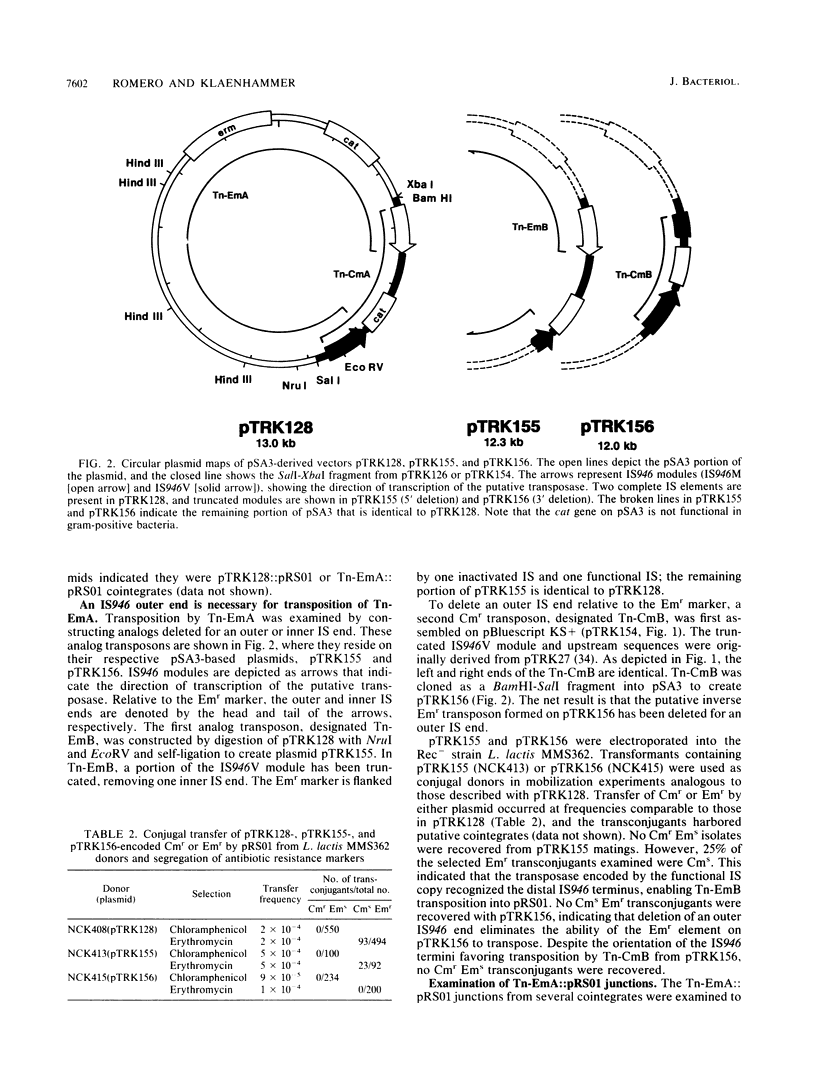
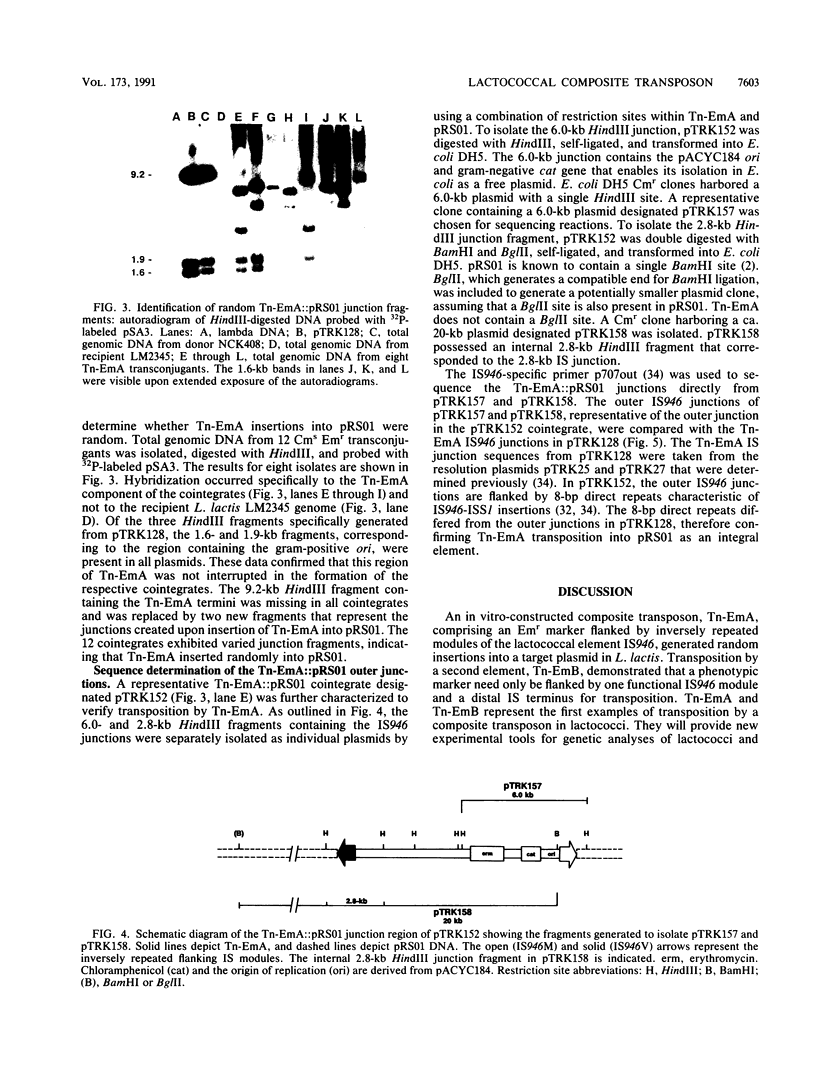
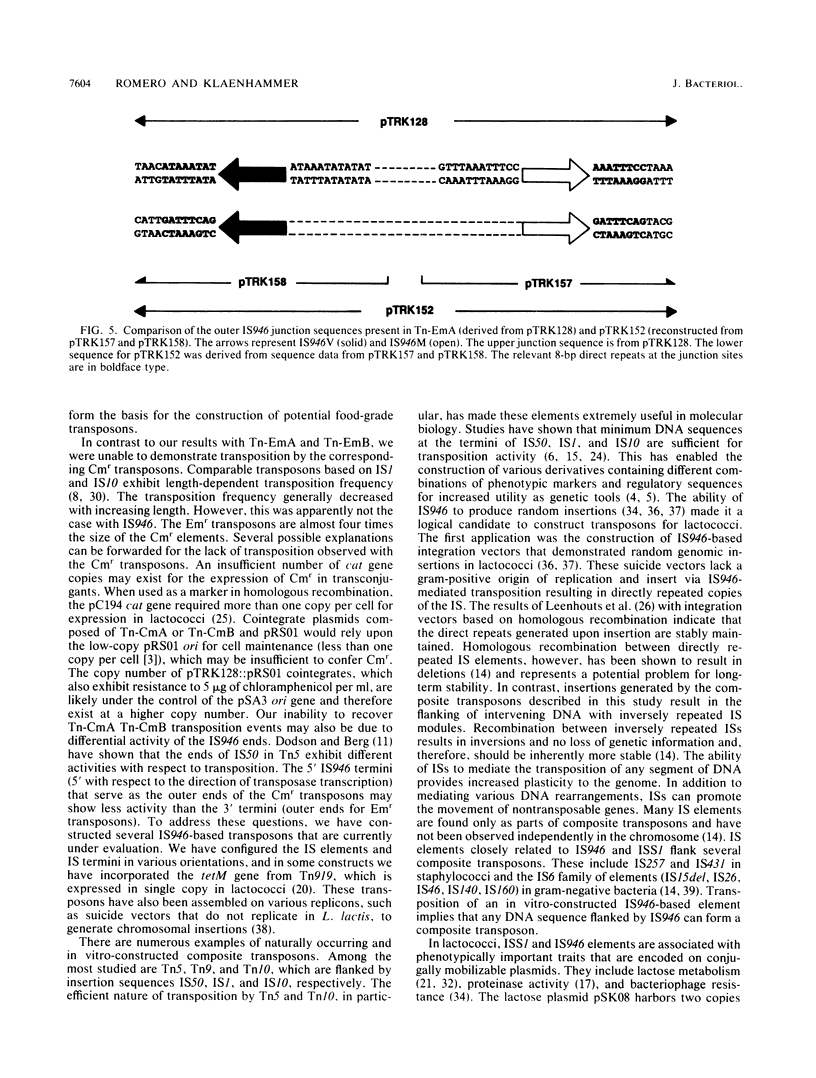
Abstract
An artificial composite transposon was constructed based on the lactococcal insertion sequence IS946. A 3.0-kb element composed of the pC194 cat gene (Cmr) flanked by inversely repeated copies of IS946 was assembled on pBluescript KS+. When subcloned into the shuttle vector pSA3 (Emr), two putative transposons were created on the recombinant plasmid pTRK128: the 3.0-kb Cmr element (Tn-CmA) and an inverse 11.5-kb Emr element (Tn-EmA). pTRK128 was electroporated into the recombination-deficient strain Lactococcus lactis MMS362, which contains the self-transmissible plasmid pRS01. An MMS362 Cmr Emr transformant was used to assay for transposition events via conjugal mobilization of pTRK128-encoded Cmr or Emr to L. lactis LM2345. Transfer of either marker alone occurred at frequencies of ca. 2 x 10(-4) per input donor. Approximately 19% of the Emr transconjugants were Cms, indicating loss of the cat gene marker. No Cmr Ems transconjugants were recovered (n = 550). Plasmid analysis showed that the Cms Emr isolates contained a single large plasmid that was determined to be a cointegrate between pRS01 and the Tn-EmA element. A 32P-labeled pSA3 probe hybridized specifically to pTRK128 sequences and revealed different junction fragments within each of the cointegrate plasmids. DNA sequence analysis of the Tn-EmA::pRS01 junctions from a representative cointegrate verified transposition by Tn-EmA. This represents the first example of a functional composite transposon in the genus Lactococcus and serves as an experimental tool and model for the genetic analyses of transposons in these organisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Genetic and physical characterization of recombinant plasmids associated with cell aggregation and high-frequency conjugal transfer in Streptococcus lactis ML3. J Bacteriol. 1984 Jun;158(3):954–962. doi: 10.1128/jb.158.3.954-962.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. In Vivo Cloning of lac Genes in Streptococcus lactis ML3. Appl Environ Microbiol. 1984 Feb;47(2):245–249. doi: 10.1128/aem.47.2.245-249.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M., Clerget M., Galas D. J. The transposition frequency of IS1-flanked transposons is a function of their size. J Mol Biol. 1982 Jan 15;154(2):229–243. doi: 10.1016/0022-2836(82)90062-6. [DOI] [PubMed] [Google Scholar]
- Dao M. L., Ferretti J. J. Streptococcus-Escherichia coli shuttle vector pSA3 and its use in the cloning of streptococcal genes. Appl Environ Microbiol. 1985 Jan;49(1):115–119. doi: 10.1128/aem.49.1.115-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd H. M., Horn N., Gasson M. J. Analysis of the genetic determinant for production of the peptide antibiotic nisin. J Gen Microbiol. 1990 Mar;136(3):555–566. doi: 10.1099/00221287-136-3-555. [DOI] [PubMed] [Google Scholar]
- Dodson K. W., Berg D. E. Factors affecting transposition activity of IS50 and Tn5 ends. Gene. 1989;76(2):207–213. doi: 10.1016/0378-1119(89)90161-3. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald G. F., Gasson M. J. In vivo gene transfer systems and transposons. Biochimie. 1988 Apr;70(4):489–502. doi: 10.1016/0300-9084(88)90085-5. [DOI] [PubMed] [Google Scholar]
- Gamas P., Galas D., Chandler M. DNA sequence at the end of IS1 required for transposition. Nature. 1985 Oct 3;317(6036):458–460. doi: 10.1038/317458a0. [DOI] [PubMed] [Google Scholar]
- Gasson M. J. In vivo genetic systems in lactic acid bacteria. FEMS Microbiol Rev. 1990 Sep;7(1-2):43–60. doi: 10.1111/j.1574-6968.1990.tb04878.x. [DOI] [PubMed] [Google Scholar]
- Haandrikman A. J., van Leeuwen C., Kok J., Vos P., de Vos W. M., Venema G. Insertion elements on lactococcal proteinase plasmids. Appl Environ Microbiol. 1990 Jun;56(6):1890–1896. doi: 10.1128/aem.56.6.1890-1896.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C., Massey I. J., Klaenhammer T. R. Rapid method to characterize lactococcal bacteriophage genomes. Appl Environ Microbiol. 1991 Jan;57(1):283–288. doi: 10.1128/aem.57.1.283-288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C., Pierce K., Klaenhammer T. R. The conjugative plasmid pTR2030 encodes two bacteriophage defense mechanisms in lactococci, restriction modification (R+/M+) and abortive infection (Hsp+). Appl Environ Microbiol. 1989 Sep;55(9):2416–2419. doi: 10.1128/aem.55.9.2416-2419.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C., Venema G., Daly C., Fitzgerald G. F. Cloning and characterization of the tetracycline resistance determinant of and several promoters from within the conjugative transposon Tn919. Appl Environ Microbiol. 1988 May;54(5):1230–1236. doi: 10.1128/aem.54.5.1230-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D. C., Novel M., Novel G. A transposon-like element on the lactose plasmid of Lactococcus lactis subsp. lactis Z270. FEMS Microbiol Lett. 1991 Jan 1;61(1):101–106. doi: 10.1016/0378-1097(91)90021-2. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Leenhouts K. J., Kok J., Venema G. Campbell-like integration of heterologous plasmid DNA into the chromosome of Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1989 Feb;55(2):394–400. doi: 10.1128/aem.55.2.394-400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenhouts K. J., Kok J., Venema G. Stability of Integrated Plasmids in the Chromosome of Lactococcus lactis. Appl Environ Microbiol. 1990 Sep;56(9):2726–2735. doi: 10.1128/aem.56.9.2726-2735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchansky J. B., Benson A. K., Atherly A. G. Construction, transfer and properties of a novel temperature-sensitive integrable plasmid for genomic analysis of Staphylococcus aureus. Mol Microbiol. 1989 Jan;3(1):65–78. doi: 10.1111/j.1365-2958.1989.tb00105.x. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Walsh P. M. Conjugal transfer of genetic information in group N streptococci. Appl Environ Microbiol. 1980 Jul;40(1):84–89. doi: 10.1128/aem.40.1.84-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisato D., Way J. C., Kim H. J., Kleckner N. Tn10 transposase acts preferentially on nearby transposon ends in vivo. Cell. 1983 Mar;32(3):799–807. doi: 10.1016/0092-8674(83)90066-1. [DOI] [PubMed] [Google Scholar]
- Polzin K. M., McKay L. L. Identification, DNA sequence, and distribution of IS981, a new, high-copy-number insertion sequence in lactococci. Appl Environ Microbiol. 1991 Mar;57(3):734–743. doi: 10.1128/aem.57.3.734-743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzin K. M., Shimizu-Kadota M. Identification of a new insertion element, similar to gram-negative IS26, on the lactose plasmid of Streptococcus lactis ML3. J Bacteriol. 1987 Dec;169(12):5481–5488. doi: 10.1128/jb.169.12.5481-5488.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D. A., Klaenhammer T. R. Characterization of insertion sequence IS946, an Iso-ISS1 element, isolated from the conjugative lactococcal plasmid pTR2030. J Bacteriol. 1990 Aug;172(8):4151–4160. doi: 10.1128/jb.172.8.4151-4160.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouch D. A., Skurray R. A. IS257 from Staphylococcus aureus: member of an insertion sequence superfamily prevalent among gram-positive and gram-negative bacteria. Gene. 1989;76(2):195–205. doi: 10.1016/0378-1119(89)90160-1. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Nicholson M. A. A method for genetic transformation of nonprotoplasted Streptococcus lactis. Appl Environ Microbiol. 1987 Aug;53(8):1730–1736. doi: 10.1128/aem.53.8.1730-1736.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]




