Abstract
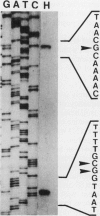
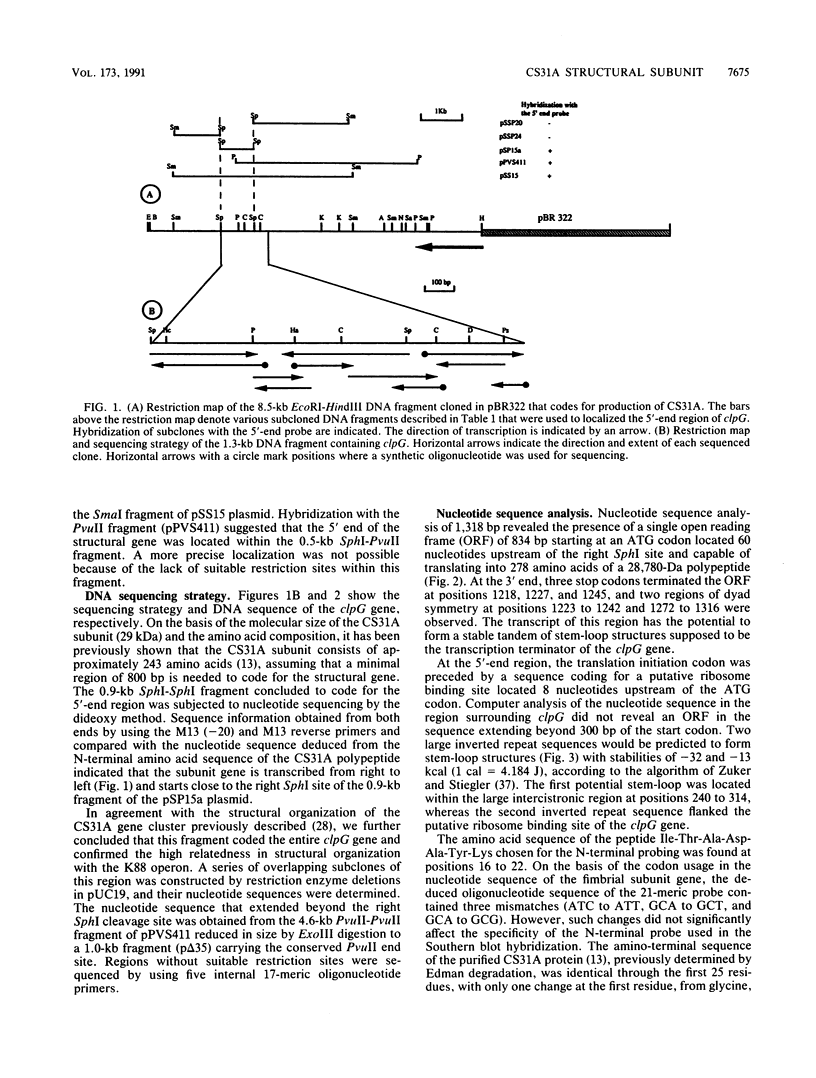
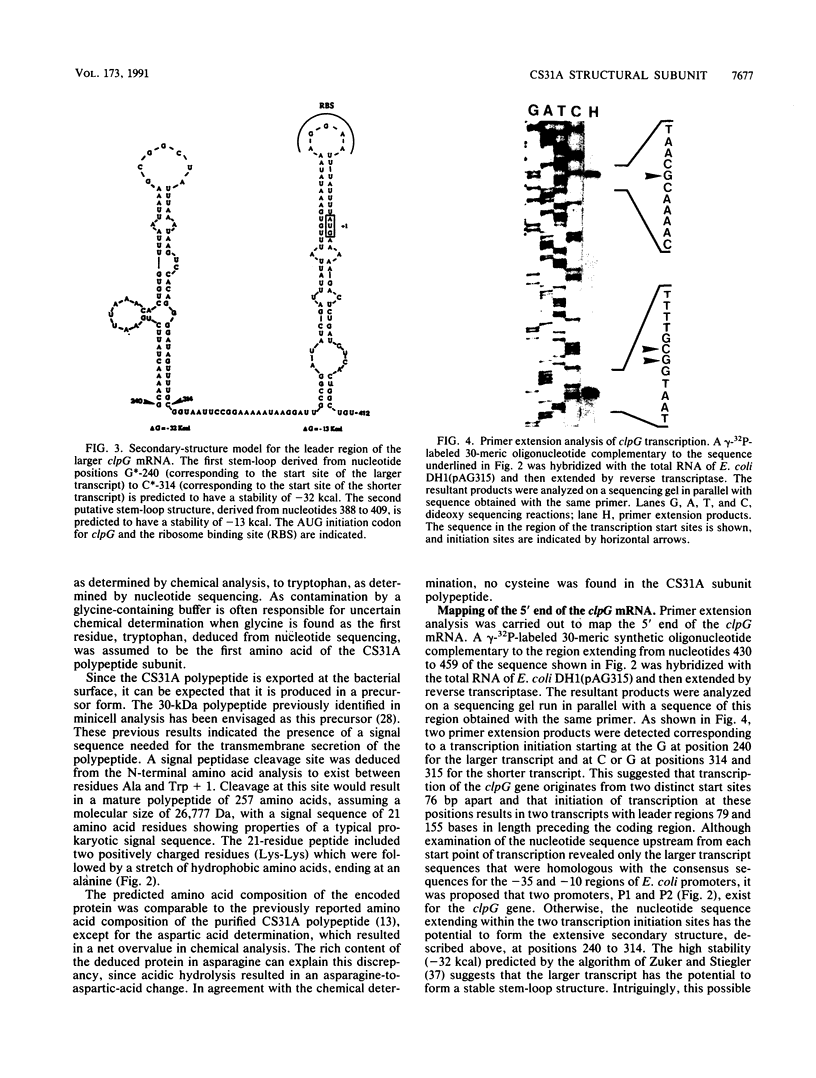
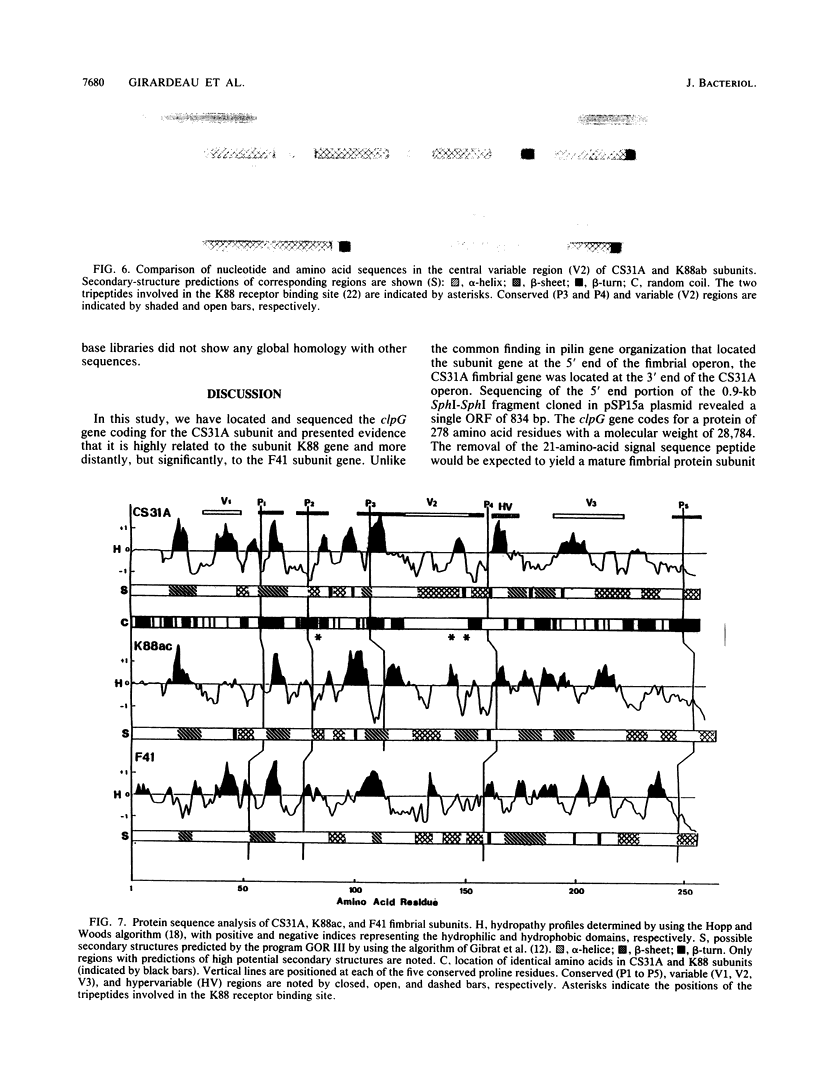
The clpG gene coding for the CS31A subunit was localized on a 0.9-kb SphI fragment from the recombinant plasmid pAG315. This was established by testing the ability of subclones to hybridize with a 17-meric oligonucleotide probe obtained from N-terminal analysis of the CS31A subunit. The nucleotide sequence of the region coding for CS31A was determined. From primer extension analysis, two initiation translation start sites were detected. Two possible promoterlike sequences were identified; the ribosome binding site and the translation terminator are proposed. Inverted repeat sequences leading to the formation of possible hairpin structures of the transcripts were found on the 5' untranslated region of clpG. The deduced amino acid composition was in close agreement with the chemical amino acid composition and sequence match with the first 25 N-terminal amino acids from the published N-terminal sequence of the purified CS31A subunit. The clpG gene codes for a mature protein of 257 amino acids with a molecular size of 26,777 Da. An obvious homology was observed when the amino acid sequence of CS31A was compared with those of K88 and F41. This homology includes five different conserved sequences of up to 19 identical amino acids, which is associated with conserved proline. An extensive change in the CS31A region homologous to that identified to contain the K88 receptor binding site might be responsible for the functional divergence between CS31A and K88.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., Moseley S. L. Escherichia coli F41 adhesin: genetic organization, nucleotide sequence, and homology with the K88 determinant. J Bacteriol. 1988 Oct;170(10):4890–4896. doi: 10.1128/jb.170.10.4890-4896.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie J. U., Reidhaar-Olson J. F., Lim W. A., Sauer R. T. Deciphering the message in protein sequences: tolerance to amino acid substitutions. Science. 1990 Mar 16;247(4948):1306–1310. doi: 10.1126/science.2315699. [DOI] [PubMed] [Google Scholar]
- Casey T. A., Moseley S. L., Moon H. W. Characterization of bovine septicemic, bovine diarrheal, and human enteroinvasive Escherichia coli that hybridize with K88 and F41 accessory gene probes but do not express these adhesins. Microb Pathog. 1990 Jun;8(6):383–392. doi: 10.1016/0882-4010(90)90025-l. [DOI] [PubMed] [Google Scholar]
- Cherifi A., Contrepois M., Picard B., Goullet P., de Rycke J., Fairbrother J., Barnouin J. Factors and markers of virulence in Escherichia coli from human septicemia. FEMS Microbiol Lett. 1990 Aug;58(3):279–283. doi: 10.1111/j.1574-6968.1990.tb13989.x. [DOI] [PubMed] [Google Scholar]
- Contrepois M., Fairbrother J. M., Kaura Y. K., Girardeau J. P. Prevalence of CS31A and F165 surface antigens in Escherichia coli isolates from animals in France, Canada and India. FEMS Microbiol Lett. 1989 Jun;50(3):319–323. doi: 10.1016/0378-1097(89)90439-4. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Dykes C. W., Halliday I. J., Read M. J., Hobden A. N., Harford S. Nucleotide sequences of four variants of the K88 gene of porcine enterotoxigenic Escherichia coli. Infect Immun. 1985 Oct;50(1):279–283. doi: 10.1128/iai.50.1.279-283.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Nakazawa A. Cyclic AMP-dependent initiation and rho-dependent termination of colicin E1 gene transcription. J Biol Chem. 1983 Jun 10;258(11):7072–7078. [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D. G., Barker W. C., Hunt L. T. The protein identification resource (PIR). Nucleic Acids Res. 1986 Jan 10;14(1):11–15. doi: 10.1093/nar/14.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibrat J. F., Garnier J., Robson B. Further developments of protein secondary structure prediction using information theory. New parameters and consideration of residue pairs. J Mol Biol. 1987 Dec 5;198(3):425–443. doi: 10.1016/0022-2836(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Girardeau J. P., Der Vartanian M., Ollier J. L., Contrepois M. CS31A, a new K88-related fimbrial antigen on bovine enterotoxigenic and septicemic Escherichia coli strains. Infect Immun. 1988 Aug;56(8):2180–2188. doi: 10.1128/iai.56.8.2180-2188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H. Behavior of Escherichia coli K antigens K88ab, K88ac, and K88ad in immunoelectrophoresis, double diffusion, and hemagglutination. Infect Immun. 1979 Mar;23(3):700–705. doi: 10.1128/iai.23.3.700-705.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers A. M., Pel H. J., Willshaw G. A., Kusters J. G., van der Zeijst B. A., Gaastra W. The nucleotide sequence of the first two genes of the CFA/I fimbrial operon of human enterotoxigenic Escherichia coli. Microb Pathog. 1989 Apr;6(4):297–309. doi: 10.1016/0882-4010(89)90103-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heuzenroeder M. W., Elliot T. R., Thomas C. J., Halter R., Manning P. A. A new fimbrial type (PCFO9) on enterotoxigenic Escherichia coli 09:H- LT+ isolated from a case of infant diarrhea in central Australia. FEMS Microbiol Lett. 1990 Jan 1;54(1-3):55–60. doi: 10.1016/0378-1097(90)90258-r. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoschützky H., Nimmich W., Lottspeich F., Jann K. Isolation and characterization of the non-fimbrial adhesin NFA-4 from uropathogenic Escherichia coli O7:K98:H6. Microb Pathog. 1989 May;6(5):351–359. doi: 10.1016/0882-4010(89)90077-6. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Jacobs A. A., Venema J., Leeven R., van Pelt-Heerschap H., de Graaf F. K. Inhibition of adhesive activity of K88 fibrillae by peptides derived from the K88 adhesin. J Bacteriol. 1987 Feb;169(2):735–741. doi: 10.1128/jb.169.2.735-741.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., Claassen I., Bakker D., Kuipers H., de Graaf F. K. Regulation and structure of an Escherichia coli gene coding for an outer membrane protein involved in export of K88ab fimbrial subunits. Nucleic Acids Res. 1986 Mar 25;14(6):2443–2457. doi: 10.1093/nar/14.6.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., Harms N., Bakker D., de Graaf F. K. Organization and expression of genes involved in the production of the K88ab antigen. Infect Immun. 1981 Jun;32(3):1155–1163. doi: 10.1128/iai.32.3.1155-1163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., Wouters C., Wijfjes A., de Graaf F. K. Construction and characterization of mutants impaired in the biosynthesis of the K88ab antigen. J Bacteriol. 1982 May;150(2):512–521. doi: 10.1128/jb.150.2.512-521.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., de Graaf F. K. Molecular biology of fimbriae of enterotoxigenic Escherichia coli. Curr Top Microbiol Immunol. 1985;118:119–138. doi: 10.1007/978-3-642-70586-1_7. [DOI] [PubMed] [Google Scholar]
- Moseley S. L., Dougan G., Schneider R. A., Moon H. W. Cloning of chromosomal DNA encoding the F41 adhesin of enterotoxigenic Escherichia coli and genetic homology between adhesins F41 and K88. J Bacteriol. 1986 Sep;167(3):799–804. doi: 10.1128/jb.167.3.799-804.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Birch-Andersen A., Duguid J. P., Stenderup J., Orskov F. An adhesive protein capsule of Escherichia coli. Infect Immun. 1985 Jan;47(1):191–200. doi: 10.1128/iai.47.1.191-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons B. L., Rathman P., Malij C. R., Oudega B., de Graaf F. K. The penultimate tyrosine residue of the K99 fibrillar subunit is essential for stability of the protein and its interaction with the periplasmic carrier protein. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):107–112. doi: 10.1016/0378-1097(90)90177-r. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]