Abstract
Background
Earlier studies from this laboratory reported that light and its spectra influence reproduction in the Indian skipper frog Rana cyanophlyctis through both ocular and extra ocular photoreception. During the course of our ongoing studies on chromotactic behaviour of the tadpoles, we noticed that tadpoles held in red light metamorphosed earlier than those held in white or other colours of light. The focus of the present study therefore was to examine the effect of red light on metamorphosis of the tadpoles.
Results
Tadpoles, both intact and blind (optectomised), held in red light metamorphosed earlier than those held in white light. Addition of melatonin to aquarium water (5 micrograms/litre) prevented the red light-induced acceleration of metamorphosis both in intact and blinded tadpoles.
Conclusion
Both ocular and extra-ocular perception of light is involved in red light-induced precocious metamorphosis. Melatonin inhibits the red light-induced acceleration of metamorphosis. The mechanism by which red light accelerates metamorphosis is not yet known. Melatonin counteracts red-light induced acceleration of metamorphosis in this tadpole.
Background
Frogs have a photosensitive pineal gland and therefore perception of light is both ocular and extra ocular [1]. Anuran amphibians are unique in possessing an extracranially located frontal organ having photoreceptor elements [2]. An earlier study from this laboratory reported that perception of light either through ocular or extra ocular (through frontal organ) pathways modulates ovarian function in the Indian skipper frog Rana cyanophlyctis [3]. Recently, in a follow up study we set up an experiment to examine chromotactic behaviour of the tadpoles. During the course of this study we noticed that tadpoles held in red light metamorphosed earlier than those held in white or other colours of light. We then focused on this finding and set out to study the effects of red light on tadpole metamorphosis in intact and blinded tadpoles. While intact tadpoles presented a model in which both ocular and extra-ocular photoreception was possible, the blinded tadpoles served as a model in which only extra-ocular photoreception was possible. We report here for the first time the effect of red light and the pineal hormone melatonin on metamorphosis of intact and blinded tadpoles of the Indian skipper frog Rana cyanophlyctis.
Results
Intact tadpoles held in red light metamorphosed within two weeks whereas those held in white light took six-eight weeks to complete metamorphosis (Fig. 1). However, addition of melatonin (5 μg/litre of water) prevented the red light-induced acceleration of metamorphosis (Fig. 2,3). Interestingly, red light accelerated metamorphosis even in blind tadpoles and melatonin prevented it when added to aquarium water (5 μg/litre of water) (Fig. 2,3). While in tadpoles held in red light the hind and forelimb buds appeared on the fifth and ninth days respectively, in tadpoles held in white light the hind and forelimbs were not yet discernable at the respective times (Fig. 1). Fully formed hind limbs appeared on seventh day and forelimbs on thirteenth day of exposure to red light. Tail resorption occurred on fourteenth day of exposure to red light. Tail resorption in the controls held in white light occurred around end of sixth week. The increase in body weights of the red light-exposed tadpoles was associated with decrease in tail lengths (Figure. 2 &3). Intact and blind tadpoles held in red light showed increase in body weight and decrease in tail length whereas those held in red light and treated with melatonin did not show increase in body weight (Figs. 2 &3). Blind tadpoles held in white light did not show significant increase in body weight compared to the intact ones held in white light. Intact tadpoles held in red light and treated with melatonin showed significant decrease in body weight and increase in tail length (Figs. 2 &3)
Figure 1.
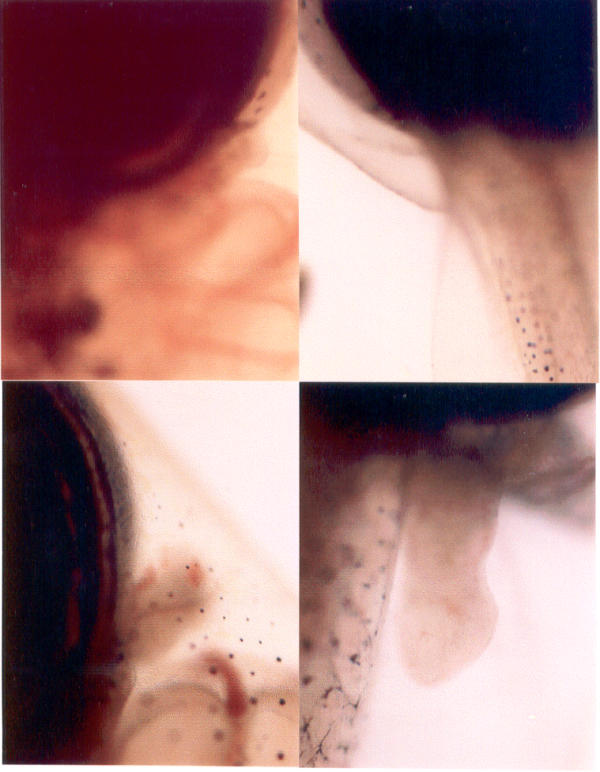
Fore and hind limb regions of the tadpoles. Fore and hind limb regions of the tadpoles held in white light (upper panel) and red (lower panel). The forelimb and hind limb buds are clearly seen in tadpoles exposed to red light.
Figure 2.
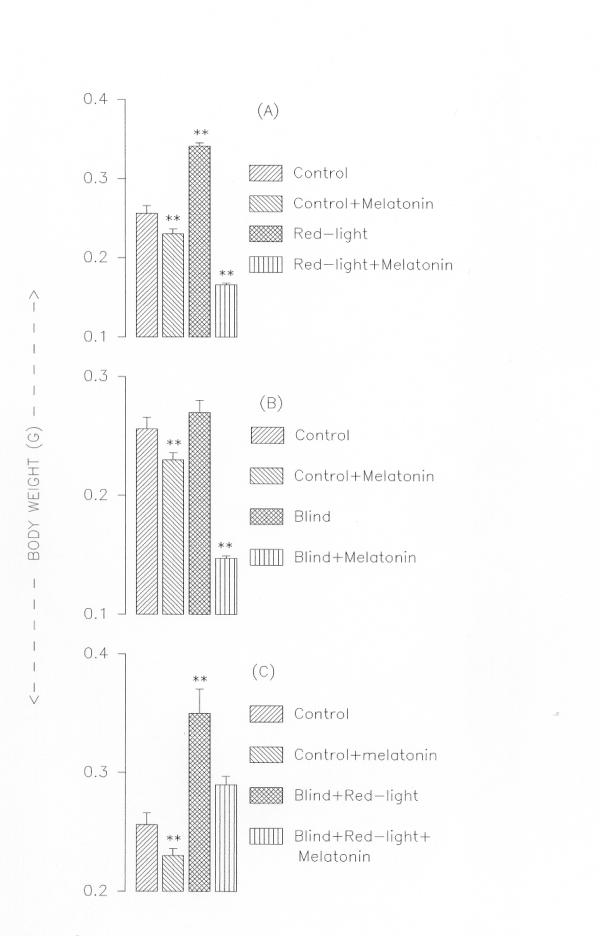
Body weights of tadpoles in different experimental groups. Panel A: Intact tadpoles held in white light, held in white light and treated with melatonin, held in red light only, held in red light and treated with melatonin. Panel B: Blind tadpoles held in white light, blind tadpoles held in white light and treated with melatonin. Panel C: Blind tadpoles held in red light, blind tadpoles held in red light and treated with melatonin. Values are Mean ± SE; **P < 0.01 control vs. treated groups
Figure 3.
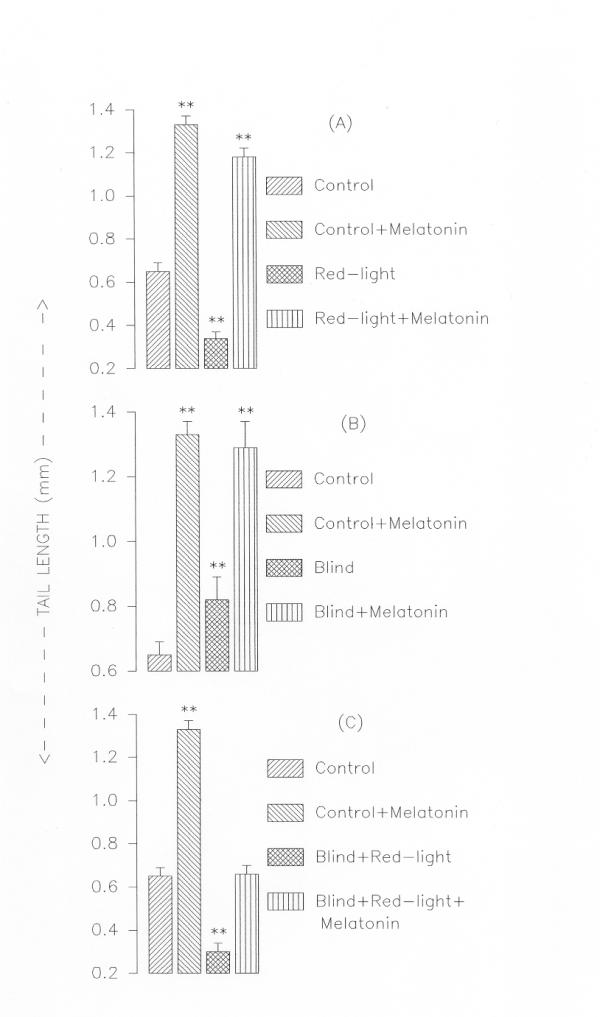
Tail lengths of tadpoles in different experimental groups. Panel A: Intact tadpoles held in white light, held in white light and treated with melatonin, held in red light only, held in red light and treated with melatonin. Panel B: Blind tadpoles held in white light, blind tadpoles held in white light and treated with melatonin. Panel C: Blind tadpoles held in red light, blind tadpoles held in red light and treated with melatonin. Values are Mean ± SE; **P < 0.01 control vs. treated groups
Discussion
Red light accelerated metamorphosis in both intact and blind tadpoles. This indicates that both ocular and extraocular perception of light influences metamorphosis. The extra ocular perception could be through the frontal organ-photoreceptor elements. At least in adult Rana cyanophlyctis frontal organ not only perceives but also differentiates different colours of light [3]. However perception of light through other photoreceptor elements located elsewhere in the skin or brain regions cannot be ruled out. In the non-mammalian vertebrates presence of multiple opsin/vitamin A-based photoreceptor elements in a variety of sites distributed both within and outside the central nervous system is documented [4,5]. It is known that extra-ocular nonvisual photoreception plays an important role in synchronising periodic functions of non-mammalian vertebrates to the environmental photoperiod [6]. What is important however is that the perception of light either through visual or non-visual photoreceptors is effective in acceleration of metamorphosis. In other words the observed effects of red light may not be associated with actually 'seeing' the colours. Even the red light-induced stimulation of ovarian function in this species occurred irrespective of whether the perception of light was ocular or non-ocular [3]. Since red light had its effect on metamorphosis even in blinded tadpoles it is necessary to study the biological importance of non-ocular photoreceptors, especially those present in the frontal organ. The frontal organ of the frog participates, in some way, in several different functions, such as neuroendocrine activities, motor responses and lateral eyes photoreception interaction [7].
The frog pineal organ is considered a predominantly "rod-type" and the frontal organ a "cone-type" photosensory organ. The presence of three kinds of pineal/frontal photoreceptors is discussed in connection with the occurrence of different photo pigments (rhodopsin/porphyropsin, iodopsin, ultraviolet and/or blue pigments) enabling the animal to discriminate by the pineal complex environmental light in various ranges of the spectrum [8]. Seasonal variation in the structure of photoreceptors of frontal organ is noticed [9]. The question regarding the functional importance of frontal organ in the biology of amphibians still remains to be understood.
The mechanism/s by which only red light or different spectra of light influence the cell biology of both ocular /and non-ocular photoreceptors need to be studied. Recent findings show that non-mammalian vertebrates possess multiple extra-retinal photoreceptors that may express novel photo pigments in these species [4,10]. It would be interesting to examine the presence of such novel photo pigments in the species.
Since both pineal and the lateral eyes produce melatonin and light regulates its production [11], it was logical to examine if this hormone is involved in the photo-induced changes on metamorphosis. Melatonin prevented red light-induced acceleration of metamorphosis in both intact and blind tadpoles. The precise mechanism of its action however is not clear, but what can be extrapolated is that melatonin may be acting through its known inhibitory influence on thyroid function [12,13]. Red light-induced acceleration of metamorphosis could be due to inhibition of melatonin production by the red spectrum of light. It would certainly be necessary to assay the hormone to support the hypothesis.
It is known that thyroid gland regulates metamorphosis in amphibians [14]. Whether the effects of light and its spectra on metamorphosis of the tadpoles are mediated by changes in thyroid physiology, directly or indirectly, needs to be ascertained. While reporting on the effects of enucleation and exposure to red light on ovarian function in Rana cyanophlyctis Joshi and Udaykumar [3] suggested that enucleation-induced ovarian stimulation was probably due to elimination of ocular source of melatonin, which is a major source of melatonin in frogs [11]. Metamorphosis of tadpoles involves a complex sequence of changes in which variations in tissue sensitivity occur during various stages of development [15]. The degree and rapidity of the changes may be the function of the state of development of the organism at the time of exposure to red light and the concentration of the thyroid hormones. Both of which may be related to the sensitivity of the tissue. Therefore, red light may either be acting via pituitary-thyroid axis or simply increase the sensitivity of the tissue to the hormone. It is not known if the colour-vision of frogs, signals for changes in neuro-endocrine function that affects metamorphosis.
Conclusions
Exposure to red light resulted in precocious metamorphosis of the tadpoles of Rana cyanophlyctis. Red light was effective in accelerating the metamorphosis both in intact and blinded tadpoles, leading to the conclusion that both ocular and extra ocular perception of light affect the process of metamorphosis. Melatonin inhibits the red light-induced acceleration of metamorphosis. The mechanism by which red light accelerates and melatonin inhibits metamorphosis is not yet known.
Methods
A clutch of eggs of R. cyanophlyctis was brought to the laboratory from the still water bodies around the Gulbarga university campus during rainy season. After hatching, the tadpoles were divided in to eight groups of ten each. The first group served as intact control and was held in white light. Second group of intact tadpoles, also held in white light, received treatment with melatonin (Sigma), which was added to aquarium water (5 μg/litre). The third group of intact tadpoles was held in red light and the fourth group, in addition to being held in red light was treated with melatonin as above. Tadpoles that formed group five were blinded (optectomized) and held in white light, whereas those forming group six were blinded and treated with melatonin as above. Tadpoles constituting seventh group were blinded and held in red light and those of the eighth group were blinded, held in red light and treated with melatonin as above. Optectomy was performed under cold anesthesia. The photoperiod either for red or white light was LD12: 12. Aquarium water was changed every alternate day. The tadpoles were fed ad-libitum with finely chopped boiled spinach leaves daily. Redlight (λ = 700–750 nm) was obtained by covering red-polythene sheet on a specially designed dome (having circumference × diameter × height = 140 × 44 × 44 cms). The wavelength was measured using a constant-deviation-spectrometer (Hilger Watts). Body weights and tail-lengths were measured at the end of the experiment. Statistical analysis used a computer programme for ANOVA. The research work was approved by the Institutional Animal Ethics Committee.
Authors' contributions
The authors contributed equally to this work. BJ wrote the paper, both the authors read the final draft.
Acknowledgments
Acknowledgements
The work used the infrastructure facilities developed with a grant from DST-FIST Govt. of India.
Contributor Information
Bhaskar N Joshi, Email: bjo.shi@sancharnet.in.
Khaja Mohinuddin, Email: khajacroma@rediffmail.com.
References
- Joshi BN, Udaykumar K. Changes in ovarian follicular kinetics in intact, blinded and parietal shielded frogs exposed to different spectra of light. Gen Comp Endocrinol. 1998;109:310–314. doi: 10.1006/gcen.1997.7034. [DOI] [PubMed] [Google Scholar]
- Vollarth L. The Pineal Organ. Heidelberg/Newyork: Springer-Verlag. 1981.
- Joshi BN, Udaykumar K. Melatonin counteracts the stimulatory effects of blinding and exposure to red light on reproduction in the skipper frog Rana cyanophlyctis. Gen Comp Endocrinol. 2000;118:90–95. doi: 10.1006/gcen.1999.7443. [DOI] [PubMed] [Google Scholar]
- Foster RG, Soni BG. Extra retinal photoreceptors and their regulation of temporal physiology. Rev Reprod. 1998;3:145–150. doi: 10.1530/revreprod/3.3.145. [DOI] [PubMed] [Google Scholar]
- Foster RG, Hankins MW. Non-rod, non cone photoreception in the vertebrtates. Prog Retin Eye Res. 2002;21:507–527. doi: 10.1016/S1350-9462(02)00036-8. [DOI] [PubMed] [Google Scholar]
- Vigh B, Manzano MJ, Zadoril A, Frank CL, Lukats A, Rohlich P, Szell A, David C. Non-visual photoreceptors of the deep brain, pineal organs and retina. Histol Histopathol. 2002;17:555–590. doi: 10.14670/HH-17.555. [DOI] [PubMed] [Google Scholar]
- Kemali M, De Santis A. The extra cranial portion of the pineal complex of the frog (frontal organ) is connected to the pineal, the hypothalamus, the brain stem and the retina. Exp Brain Res. 1983;53:193–196. doi: 10.1007/BF00239412. [DOI] [PubMed] [Google Scholar]
- Vigh B, Vigh-Teichmann I. Three types of photoreceptors in the pineal and frontal organs of frogs: Ultra structure and opsin immunoreactivity. Arch Histol Jpn. 1986;49:495–518. doi: 10.1679/aohc.49.495. [DOI] [PubMed] [Google Scholar]
- Guglielmotti V, Vota-Pinardi U, Fiorino L, Sada E. Seasonal variations in the frontal organ of frog: Structural evidence and physiological correlates. Comp Biochem Physiol A. 1997;116:137–141. doi: 10.1016/S0300-9629(96)00165-X. [DOI] [PubMed] [Google Scholar]
- Foster RG, Follet BK. The involvement of a rhodopsin-like photopigment in the photoperiodic response of the Japanese quail. J Comp Physiol A. 1985;157:519–528. [Google Scholar]
- Delgado MJ, Vivien Roels B. Effect of environmental temperature and photoperiod on the melatonin levels in the pineal, lateral eyes and plasma of the frog Rana perezi : Importance of ocular melatonin. Gen Comp Endocrinol. 1989;75:46–53. doi: 10.1016/0016-6480(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. The pineal and its hormone in the control of reproduction in mammals. Endocrine Rev. 1980;1:109–131. doi: 10.1210/edrv-1-2-109. [DOI] [PubMed] [Google Scholar]
- Vriend J. Evidences for pineal gland modulation of the neuroendocrine-thyroid axis. Neuroendocrinol. 1983;36:68–78. doi: 10.1159/000123439. [DOI] [PubMed] [Google Scholar]
- Allen BB. The results of extirpation of the anterior lobe of the hypophysis and of thyroid of Rana pipiens larvae. Science. 1916;44:755–758. doi: 10.1126/science.44.1143.755. [DOI] [PubMed] [Google Scholar]
- Swingle WW. Thyroid and amphibian metamophosis. J Exptl Zool. 1922;36:397–421. [Google Scholar]


