Abstract
Ly-6C belongs to the Ly-6 family of glycosyl phosphatidylinositol-anchored surface glycoproteins and is expressed on a subset of mature CD8+ T cells. Ly-6C ligation can mediate T cell activation and causes interleukin 2 secretion in cytolytic T cell clones. We characterize herein a new mAb 1G7.G10 against Ly-6C that recognizes an epitope involved in lymphocyte adhesion and in lymphocyte homing. Pretreatment of lymph node lymphocytes and of purified CD8+ T cells (but not of lymphocytes depleted of CD8+ T cells) with 1G7.G10 reduced their in vitro binding to lymph node high endothelial venules by 28% and 34%, respectively. This effect was bypassed by cross-linking Ly-6C molecules with 1G7.G10 and a second-step antibody. The in vivo homing of (donor) CD8+ T lymphocytes to lymph nodes was reduced by Ly-6C blocking with 1G7.G10 (whole antibody) or with its fragments [F(ab) or F(ab)2] by 20% or by 32% and 48%, respectively. Cross-linking of Ly-6C in vitro induced very late antigen-4 and lymphocyte function-associated antigen 1-mediated aggregation of CD8+ T cells, suggesting that ligand binding to Ly-6C leads to activation of integrins. This activation may facilitate homing of Ly-6C+ CD8+ T cells in vivo.
Ly-6 is a family of closely related murine glycoproteins that are mainly expressed by cells of the hematopoietic lineage. The Ly-6 locus encodes at least seven structurally related molecules that represent different serotypes (Ly-6A/E, Ly-6B, Ly-6C, Ly-6D, Ly-6F, Ly-6G, and ThB) (1, 2). These Ly-6 specificities vary in their exact lymphoid and myeloid cell distribution and have, therefore, been studied extensively as hematopoietic differentiation antigens. Although the exact function of many of the Ly-6 molecules is still obscure, evidence suggests that these molecules participate in signaling steps during immune responses. (i) Ly-6A/E can mediate T cell activation independently of other soluble or cell-mediated signals, and Ly-6C mediates at least costimulatory signals for T cell activation (3, 4). (ii) T cell Ly-6A/E and Ly-6C can be up-regulated by interferons, including interferon γ (5), and by CD3 complex stimulation (6). The expression of Ly-6C on lymphocytes in Ly-6.1 mouse strains is restricted to a subset of peripheral CD8+ T lymphocytes (≈50% positive) (1), but also monocytes, macrophages, a subset of bone marrow mononuclear cells, and endothelial cells of small vessels express variable levels of Ly-6C (7).
Lymphocyte recirculation and homing are a prerequisite for effective distribution of the whole repertoire of antigen-responsive lymphocytes within the whole body (8). A key event in lymphocyte homing is the interaction of the lymphocyte with vascular endothelial cells (ECs), which can be viewed as a stepwise process (9). Many of the molecules involved in the lymphocyte–EC interaction have been characterized in detail. The first step is mediated by lymphocyte (L-selectin, peripheral node homing receptor) and endothelial selectins (E- and P-selectin) (10) or, alternatively, by α4-integrins (α4/β7, mucosal homing receptor, and α4/β1) (11) and is followed by firm adhesion of the lymphocyte to ECs mediated by β2- or, again, α4-integrins (12, 13). Before the integrin can mediate firm adhesion, it must be activated, which requires that the cell receives an appropriate signal. This signal can come from the binding of a membrane-bound molecule (for review, see ref. 9) such as a selectin (14) or a soluble molecule, many of which belong to the family of chemokines (15, 16), to leukocyte surface receptors. Some stimuli act selectively on a lymphocyte subset and enhance selective recruitment of certain lymphocytes (17). However, selective recruitment of all different lymphocyte subsets is still not completely explicable by the currently known chemokines and may, therefore, also require some other molecules.
To study molecules that mediate lymphocyte homing, we made new mAbs by using purified blood vessel fragments as the source of antigen and selected a panel of mAbs that were reactive against vascular endothelium. We report herein one of those antibodies that reacts with vascular endothelium and also with a subset of lymphocytes and that was verified as an antibody specifically recognizing Ly-6C. It was used to study the role of Ly-6C in lymphocyte and endothelial cell interactions in vitro and in vivo. Our data imply that Ly-6C participates in adhesive interactions of Ly-6C+ CD8+ T lymphocytes and may, therefore, be required for subset-specific homing of these cells.
MATERIALS AND METHODS
Production of Anti-Ly-6C mAb 1G7.G10 and Its Fragments.
The anti-Ly-6C mAb 1G7.G10 (and another anti-Ly-6C mAb 5E9) was selected from a new panel of mAbs that reacted with the endothelium of blood vessels. Since our principal aim was to study lymphocyte–EC interaction in inflammation, these mAbs were generated by immunizing a male Sprague–Dawley rat with purified vessel fragments prepared from the pancreases of nonobese diabetic mice displaying lymphocytic infiltration in pancreatic islets. Briefly, pancreases were enzymatically digested with collagenase (collagenase P; Boehringer, Mannheim) and DNase (DNase I, grade II, Boehringer), filtered through a nylon mesh (110-μm pore size) and layered on a continuous Percoll gradient. After centrifugation (1,000 × g for 10 min), the tissue had usually sedimented to four visible layers. The layers were collected, washed in RPMI 1640 medium, and checked under an inverted microscope. The second uppermost layer contained mostly small fragments of blood vessels.
After three immunizations with fresh and sonicated vessel fragments, the rat was killed and popliteal lymph node lymphocytes were fused with NS-1 myeloma cells. Growing hybridomas were tested for reactivity with vascular endothelium in immunohistochemistry, and positive hybridomas were cloned and chosen for further studies. One of the clones was termed 1G7.G10 (subsequently referred to as G10) and verified as an anti-Ly-6C mAb. The mAb 5E9 that also reacts with Ly-6C was used in some experiments as another Ly-6C mAb. The mAb 2E8 was also generated along with these two anti-Ly-6C mAbs and was used as an isotype-matched irrelevant control mAb in the high endothelial venule (HEV) binding and in vivo homing experiments. It reacts with an unknown molecule expressed at the basal aspect of blood vessels and with septal-like structures in various organs. F(ab) fragments were prepared from G10 by enzymatic cleavage of purified mAb using ficin (Sigma) as described (18), and F(ab)2 fragments were prepared from purified G10 and 2E8 by enzymatic cleavage with preactivated papain.
Immunohistochemistry and Flow Cytometry.
Cryocut sections from multiple organs of NOD and BALB/c mice were stained with mAb G10 by an immunoperoxidase method as described (19). Two-color immunofluorescence staining for flow cytometry was performed by serial incubation of the cells with G10, fluorescein isothiocyanate (FITC)-conjugated second-step antibody and a directly phycoerythrin-conjugated anti-CD4 mAb (PharMingen). Alternatively, biotinylated G10, streptavidin-phycoerythrin and a directly FITC-conjugated anti-CD8 mAb (Becton Dickinson or PharMingen) or anti-B220 mAb (TIB-146, American Type Culture Collection, ATCC) were used. To detect Mac-1 (CD11b), cells were incubated with anti-Mac-1 mAb (TIB-128, ATCC), FITC-conjugated second-step antibody, biotinylated G10, and streptavidin-phycoerythrin. mAbs G10 and TIB-210 (anti-CD8α, ATCC) were biotinylated by using N-hydroxy-succinimide (NHS)-biotin (Calbiochem).
Immunoprecipitation and Blotting of Ly-6C.
BALB/c spleen cells were lysed for 1 h in ice-cold 1% Nonidet P-40 in PBS. After centrifuging the lysate at 12,000 × g for 30 min, the supernatant was collected and used as the source of the antigen for immunoblotting. Briefly, an aliquot of this material was electrophoresed by SDS/PAGE under nonreducing conditions, transferred to nitrocellulose (Hybond ECL, Amersham), and reacted first with the anti-Ly-6C mAb Al-21 (PharMingen), G10, 5E9, or an isotype-matched control mAb and then with a peroxidase-conjugated second-step Ab (goat anti-rat IgG; Dako). The reaction was detected by enhanced chemiluminescence (ECL, Amersham).
For preabsorption of Ly-6C reactivity, immunoprecipitations were carried out by three successive incubations of the lysate with G10, 5E9, or an isotype-matched control mAb coupled to protein G-Sepharose beads (Pharmacia). The preabsorbed lysate was then subjected to immunoblotting as above.
In Vitro HEV Binding Assays.
Lymphocyte binding to HEVs was assessed by the frozen section assay essentially as described (20, 21). Briefly, freshly isolated lymphocytes from BALB/c lymph nodes were incubated on frozen sections of BALB/c lymph nodes in RPMI 1640 medium with 5% fetal calf serum and Hepes and allowed to adhere for 30 min under a constant rotation at 60 rpm at +7°C. Adherent cells were fixed in 1% glutaraldehyde/PBS.
To test the effect of an antibody on the HEV-binding of lymphocytes, either the sections (HEVs) or the lymphocytes were pretreated with saturating concentrations of the desired mAb or a control mAb for 20 min, washed once, and used for the assay. The slides (two or three slides with four to six tissue sections for each pretreatment) were read by dark-field microscopy and the number of HEVs (≈300 per pretreatment) and cells bound to these HEVs were counted. Results are expressed as relative binding ratios, which represent the relative number of lymphocytes bound to HEVs after various pretreatments compared with the number of lymphocytes bound to HEVs after a control pretreatment. The control pretreatment was similar to the pretreatment under study except that the primary anti-Ly-6C antibody was replaced with the control mAb 2E8 or TIB-218 (anti-CD18), which recognizes an epitope on CD18 not involved in lymphocyte function-associated antigen 1 (LFA-1) function (22).
To further test the specificity of the inhibition achieved by G10 pretreatment, CD8+ T lymphocytes (including all Ly-6C+ cells) were either removed by depletion or enriched by negative selection with magnetic beads (Dynabeads, Dynal, Oslo, and MACS, Miltenyi Biotec, Auburn, CA). For depletion, the cells were incubated with anti-CD8 mAb TIB-210 and anti-rat IgG-conjugated Dynabeads resulting constantly in depletion rates of more than 90%. For CD8+ T cell enrichment, lymph node lymphocytes were reacted with anti-L3T4 and anti-B220-MACS beads, which resulted in efficient removal of CD4+ T cells and B cells after purification steps (the remaining lymphocytes were typically ≥95% pure CD8+ T cells).
Lymphocyte Homing in Vivo.
Lymphocyte homing efficiency after mAb treatments was determined by flow-cytometric analyses of lymph node lymphocytes isolated from mice that had received donor lymphocytes intravenously. Briefly, lymph-node lymphocytes were isolated from 6- to 8-week-old BALB/c mice and fluorescently labeled with 5-chloromethyl fluorescein diacetate (Molecular Probes) or with PKH-26 (Sigma). Labeled lymphocytes were then incubated with mAb G10 (anti-Ly-6C) or mAb 2E8 (10 × 106 cells and 10 μg of mAb per ml of medium), washed, and injected (10 × 106 cells) into the tail vein of unmanipulated recipients; the mice were killed after 30 min and their lymph nodes were collected. In other experiments, unlabeled BALB/c lymphocytes (H-2Kd) were used. After mAb pretreatment, they were injected into SJL mice (H-2Ks) and after recirculation, only H-2Kd-positive (donor) lymphocytes were analyzed by using an allospecific mAb (FITC-conjugated anti-H-2Kd, PharMingen). In all experiments, the cells were stained after isolation for flow cytometry to detect the injected CD4 and/or CD8 T cells. This was done either with biotinylated anti-CD8 mAb (TIB-210) and phycoerythrin-conjugated streptavidin (after 5-chloromethyl fluorescein diacetate) or, alternatively, with FITC- (after PKH) or phycoerythrin- (after H-2Kd-FITC) conjugated anti-CD8 mAb (Lyt2FITC, Becton Dickinson; or Lyt2PE, PharMingen) or with phycoerythrin-conjugated (after H-2Kd-FITC) anti-CD4 mAb (L3T4PE, PharMingen). The percentage of CD8+ (or, of CD4 +) lymphocytes among fluorescent (injected) lymphocytes was measured, and the percentage of different T cell populations in cells treated with a control antibody was arbitrarily set as 100% (control homing). Cells were analyzed by using a FACScan flow cytometer and lysys ii software (Becton Dickinson).
Aggregation Assays.
To determine whether Ly-6C delivers signals that can increase lymphocyte adhesiveness, Ly-6C molecules were cross-linked by mAb G10 and anti-rat IgG (Caltag, San Francisco, CA) in an in vitro assay using lymphocyte aggregation as the readout. In all assays, CD8+ T cells were purified by negative selection with MACS antibodies as described for HEV binding assays. Purified CD8+ T cells were incubated for 20 min at +7°C first with mAb G10 or other mAbs (as indicated in Fig. 5) at 40 μg/ml in RPMI, then with anti-rat IgG, and finally resuspended in RPMI 1640 medium supplemented with 5% fetal calf serum (culture medium). To test the dependence of aggregation on various known adhesion molecules, cells were incubated another 20 min in RPMI with function-blocking mAbs before resuspending the cells in culture medium. These mAbs were TIB-237 against LFA-1 (CD11a), CRL-1878 against intercellular adhesion molecule 1 (ICAM-1) and CRL-1911 (PS/2) against α4-integrins (ATCC), Fib504 (provided by E. C. Butcher, Stanford University) against β7-integrin and a polyclonal rabbit anti-β1-integrin antiserum (provided by J. Heino, Turku University, Finland). As nonfunctional control antibodies, we used TIB-218 (ATCC) against an epitope of CD18 not involved in LFA-1-mediated functions (22) and a polyclonal rabbit anti-α1-integrin antiserum (provided by J. Heino). Cells were plated at 3 × 105 cells per 100 μl in each well on flat-bottomed 96-well plates that were spun at 1,000 × g for 1 min to bring the cells into close contact and incubated at +37°C for 2–3 h. Alternatively, cells were plated on round-bottomed 96-well plates to bring the cells into contact and incubated similarly. After that, cells and aggregates were fixed with glutaraldehyde added to each well at 1% final concentration. Cells from round-bottomed wells were transferred to flat-bottomed wells for reading. Reading was done at ×200 magnification without knowledge of the identity of the well.
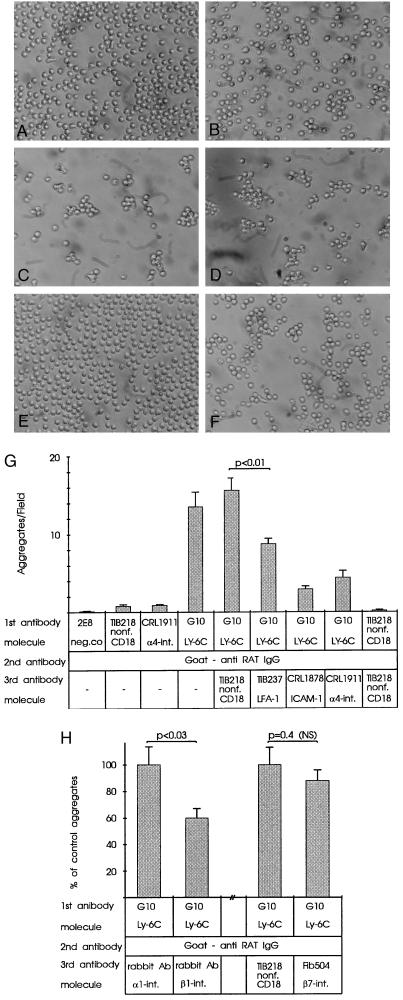
Figure 5.
Aggregation of purified CD8+ T cells after Ly-6C cross-linking is mediated by integrins. (A–F) Photomicrographs of cells fixed after 3-h incubations following mAb treatments. (A) Negative control + anti-rat IgG. (B) Nonf. anti-CD18 + anti-rat IgG (Nonf. = antibody reacts with a nonfunctional epitope of the molecule). (C) G10 + anti-rat IgG. (D) G10 + anti-rat IgG + nonf. anti-CD18. (E) G10 + anti-rat IgG + anti-ICAM-1. (F) G10 + anti-rat IgG + anti-α4-integrin. (Original magnification, ×200). (G and H) Aggregation values of CD8+ T cells after cross-linking of Ly-6C. Bars represent the aggregates per microscopic field (G) and the percentage of control aggregates (H) in three (G) and two (H) experiments, in which six fields were counted in each of duplicate or triplicate wells for each treatment (mean ± SEM).
RESULTS
mAb G10 Recognizes Ly-6C.
mAb G10 was generated by using vessel fragments from the pancreases of nonobese diabetic mice as the immunogen. It was selected for further studies by screening antibody reactivity with vascular endothelium. On endothelium, mAb G10 reactivity was strongest in small-sized vessels that stained at a similar level in the brain, heart, kidney glomeruli, lung, spleen, and lymph nodes. In addition, some larger vessels stained weakly and in lymph nodes, some HEVs also occasionally stained weakly. Some lymph node and spleen cells were also stained in BALB/c mice but this staining was not seen in NOD mice (data not shown). The reactivity of the mAb 5E9 in immunohistochemistry was similar to that of G10 but a flow cytometry analysis of lymphocytes after serial incubations with this mAb and G10 showed, however, that mAb 5E9 recognized a distinct but slightly overlapping epitope on lymphocytes.
In flow cytometry, G10 stained 50% of CD8+ BALB/c lymph node T cells (Fig. 1A), both CD8+ and Mac-1+ spleen cells (data not shown) and the majority of large granular bone marrow cells (Fig. 1B), whereas in NOD mice only a small proportion of large granular bone marrow cells (Fig. 1B) stained positively. This is consistent with the previous data on the differences in the expression of Ly-6C in lymphoid and myeloid cells of BALB/c and NOD mice (23, 24).
Figure 1.
mAb G10 reacts with Ly-6C+ cells. (A) G10 expression in BALB/c (Ly-6.1) lymph node cells. The G10bright cells in the histogram are mostly CD8+ cells (Insets) that in turn are divided into G10+ and G10− cells. (B) In BALB/c bone marrow, large granular cells (region R1) are divided into three populations with distinct intensities, most cells being G10moderate. In NOD bone marrow, large granular cells are mostly G10negative and very few are G10positive.
To verify the reactivity of mAbs G10 and 5E9 with Ly-6C, BALB/c spleen cell lysate was preabsorbed with either of these mAbs or with an isotype-matched control mAb and then probed for remaining Ly-6C reactivity by immunoblotting (Fig. 2). The known anti-Ly-6C mAb Al-21 and mAbs G10 and 5E9 reacted with a molecule with a 12- to 14-kDa molecular mass and the Al-21 reactivity was removed by preabsorption of the lysate with either of the mAbs G10 or 5E9. In contrast, preabsorption with an isotype-matched control mAb did not affect Al-21 reactivity. Therefore, the molecule recognized by G10 and 5E9 is Ly-6C.
Figure 2.
Immunoprecipitation and Western blotting of Ly-6C. BALB/c spleen cell lysates were electrophoresed in 5–12.5% SDS/PAGE gels under nonreducing conditions, transferred to nitrocellulose, and detected by the ECL method using mAbs Al-21 (against Ly-6C) and G10, 5E9, or a control mAb (first four lanes). Alternatively, the same lysate was preabsorbed with mAbs G10, 5E9, or a control mAb before SDS/PAGE and probing with the Ly-6C mAb Al-21 (last three lanes).
mAb G10 Inhibits in Vitro HEV Binding of Lymphocytes.
When preincubated with mAb G10, lymphocyte binding to lymph node HEVs was reduced by 28% compared with lymphocyte binding after preincubation with the control mAb 2E8 (P = 0.002; Fig. 3A). This effect was abolished when the Ly-6C molecules were cross-linked by preincubation of the cells first with G10 and then with a polyclonal anti-rat IgG antibody (Fig. 3A), suggesting that this treatment may have mimicked the effect that the binding of the natural ligand would have had if Ly-6C mediates activating signals to other adhesion molecules. Preincubation of the tissue section with mAb G10 did not affect lymphocyte binding significantly (mean number of bound lymphocytes was 94% of control).
Figure 3.
Effect of mAb G10 pretreatment of lymphocytes on their in vitro binding to lymph node HEVs. (A) The mAb G10 inhibits lymphocyte binding to HEVs but this inhibition is abolished when the mAb G10 epitope is cross-linked with a second-step antibody. (B) Binding of CD8+ T cell-depleted lymphocytes to HEVs after mAb G10 pretreatment is similar to control. (C) Binding of purified CD8+ T cells is inhibited by mAb G10. Bars represent the mean ± SEM of the relative binding ratios observed in 10 (A) and in 2 (B and C) individual experiments. Note that the inhibition rate in each case must be considered against the proportion of Ly-6C+ cells in that case.
To further establish the specificity of the effect of G10 pretreatment, the same HEV assay was performed with G10-pretreated lymphocytes that were depleted of CD8+ T cells (including all Ly-6C+ cells) or of CD4+ T cells and B cells. Depletion of CD8+ T cells instead of only Ly-6C+ CD8+ cells was chosen because the anti-Ly-6C mAbs did not deplete all Ly-6C+ cells appropriately. G10 pretreatment had no effect on the HEV binding of CD8+ T cell-depleted lymphocytes (Fig. 3B) but reduced HEV binding of purified CD8+ T cells significantly (Fig. 3C). This shows that the inhibitory effect of mAb G10 treatment was specific for CD8+ (thus for Ly-6C+) lymphocytes.
Inhibition of in Vivo Lymphocyte Homing by mAb and F(ab) or F(ab)2 G10.
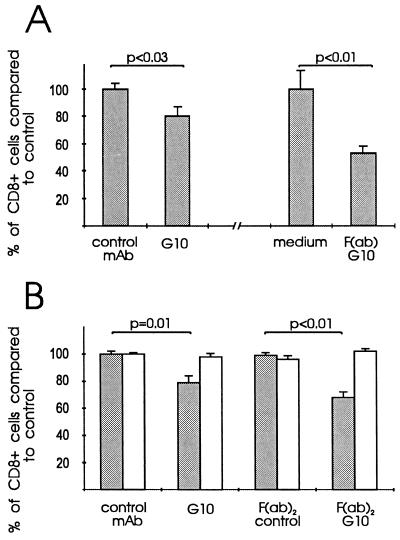
The effect of G10 pretreatment on lymphocyte homing was determined by analyzing the percentage of CD8+ T cells (50% of which are Ly-6C +) among fluorescently labeled cells that had homed to lymph nodes after being injected i.v. to unmanipulated mice 30 min earlier. When pretreated with whole mAb G10 IgG, the homing of CD8+ T cells was reduced by a mean of 20% when compared with control mAb. Pretreatment of the cells with F(ab)2 or F(ab) G10 reduced the homing of CD8+ T cells to lymph nodes by 32% and 48% (Fig. 4). This effect was specific for CD8+ T cells since the percentages of CD4+ T cells were similar to their controls.
Figure 4.
Effect of mAb G10 pretreatment on the homing of CD8+ lymphocytes. (A) In two sets of experiments, fluorescently labeled lymphocytes were pretreated with mAb G10 or an isotype-matched IgG1 control mAb (2E8) or alternatively, with F(ab)G10 or medium alone. (B) In separate experiments, lymphocytes were pretreated with whole mAbs or their F(ab)2 fragments. Pretreated lymphocytes were injected intravenously and analyzed from lymph nodes after a 30-min recirculation and homing period. Shaded bars, CD8+; open bars, CD4+ T cells (to control specificity). The y axis indicates the percentage of CD8+ (or, of CD4+) T cells compared with that in control samples, which was arbitrarily set as 100% (=control homing).
Ly-6C Signals Activation of β1- and β2-Integrins.
To determine whether Ly-6C can signal activation of integrin-type adhesion molecules, we stimulated Ly-6C molecules by cross-linking them (with mAb G10 and a second-step antibody) and studied its effect on integrin-mediated adhesiveness in an assay using lymphocyte aggregation as the readout. Cross-linking caused aggregation of purified CD8+ T cells. When lymphocytes were preincubated with an antibody against LFA-1 (TIB-237), ICAM-1, or α4-integrin after cross-linking Ly-6C, lymphocyte aggregation diminished markedly (Fig. 5 A–G).To determine whether the inhibition obtained with anti-α4-integrin mAb reflected β1- or β7-integrin function, we used function-blocking antibodies against these molecules in separate assays. These assays revealed that aggregation was dependent on β1-integrin but not on β7-integrin (Fig. 5H).
DISCUSSION
In this study, a novel function for Ly-6C is described. Our initial aim was to examine endothelial adhesion molecules mediating lymphocyte homing, and to this end, we generated a panel of mAbs reactive with vascular endothelium that could inhibit lymphocyte binding to HEVs in the frozen section assay. mAb G10 described herein inhibited lymphocyte binding to HEVs. By immunoprecipitations and its expression pattern, mAb G10 was subsequently identified as a mAb against Ly-6C. Although Ly-6C is also expressed on endothelium, and weakly on some HEVs as well, only the lymphocyte Ly-6C was found to regulate endothelial adhesion of the Ly-6C+ lymphocyte population and its in vivo homing.
A number of studies have focused on the function of Ly-6 molecules on lymphocytes. Of these, Ly-6C is known to function in synergy with the CD3–T cell receptor complex (3, 25–28) or even alone (29) to signal T cell responses. T cell activation via Ly-6 leads to calcium influx (27) and, like several glycosyl phosphatidylinositol (GPI)-anchored glycoproteins, the Ly-6 molecules may be linked to tyrosine kinases (30). One reported consequence of Ly-6 signaling in T cells is an increase in the expression of the interleukin 2 receptor and secretion of interleukin 2 (27). The evolutionarily conserved Ly-6 molecules may also be required in some other intracellular or intercellular events besides T cell activation. According to the present study, Ly-6C molecules also regulate endothelial and homotypic adhesion and in vivo homing of a subset of CD8+ T cells. Recently, evidence for the involvement of other Ly-6 molecules in adhesive interactions between cells has emerged. Accordingly, Ly-6A/E mediates homotypic adhesion between transgenic thymocytes made to express high levels of the molecule (31) and the human homologue for ThB mediates adhesion between epithelial cells (32).
In the in vivo homing experiments, F(ab) G10 inhibited the homing of CD8+ lymphocytes by 48%, which is more than the 32% and 20% inhibition rates achieved with F(ab)2 and the whole mAb, respectively. This may be because in the in vivo conditions, a divalent mAb bound to the Ly-6C molecules may to some extent also activate the Ly-6C molecule instead of solely blocking its function. Therefore, the inhibition achieved by the F(ab) G10 may best reflect the proportional contribution that Ly-6C would have in the homing of CD8+ T cells.
Lymphocyte attachment to vascular endothelium is a stepwise process that requires coordinated interactions between several molecules and their ligands during the course of lymphocyte tethering and rolling, firm adhesion, and transendothelial migration (9, 15, 16, 33). The exact step of Ly-6C involvement in lymphocyte homing cannot be directly predicted from the homing experiments performed and such predictions are further complicated by the fact that the endothelial or other ligand/ligands of Ly-6C are not known. However, our in vitro experiments in which we inhibited the homotypic aggregation of purified CD8+ T cells after cross-linking Ly-6C indicate that Ly-6C acts as a molecule that signals activation of other adhesion molecules. Function-blocking antibodies against LFA-1, ICAM-1, α4-integrin, and β1- but not β7-integrin revealed that the aggregation was dependent on LFA-1 and very late antigen-4 (VLA-4) and verified that Ly-6C signals activation of both β1- and β2-integrins.
Compared with chemokine receptors that are coupled to G proteins (15), binding of the ligand (supposedly not a chemokine) to the Ly-6C molecule on lymphocytes may lead to different signal transduction pathways. Ligand (or mAb) binding to the GPI-linked molecules may activate the inositol phospholipid pathway (34) resulting in the formation of biologically active inositol trisphosphate and an increase in cytosolic free calcium (28). In addition, ligation of GPI-linked molecules may cause internalization of the complex and enzymatic cleavage of the lipid tail that may lead to the release of diacylglycerol and subsequent activation of protein kinase C (PKC) (25). At least in platelets, activation of the integrin adhesion molecule αIIbβ3 involves both an increase in intracellular calcium content and activation of PKC (35). Therefore, it is possible that other integrins may also be activated via similar pathways and as a consequence of the initial ligation of a GPI-anchored surface molecule like Ly-6C.
In conclusion, Ly-6C plays a role in the endothelial adhesion and in vivo homing of Ly-6C+ T cells. The role of Ly-6C in these events is to signal activation of integrin-type adhesion molecules. As Ly-6C is expressed only on a subpopulation of lymphocytes, Ly-6C represents a potentially important signaling molecule regulating the homing of these cells in a subtype-specific manner.
Acknowledgments
This work was supported by the Foundation for Diabetes Research, Finland, the Novo-Nordisk Fond, and the Finnish Academy.
ABBREVIATIONS
- EC
endothelial cell
- GPI
glycosyl phosphatidylinositol
- HEV
high endothelial venule
- LFA
lymphocyte function-associated antigen
- FITC
fluorescein isothiocyanate
- ICAM-1
intercellular adhesion molecule 1
References
- 1.Shevach E M, Korty P E. Immunol Today. 1989;10:195–200. doi: 10.1016/0167-5699(89)90324-1. [DOI] [PubMed] [Google Scholar]
- 2.Fleming T J, Fleming M L, Malek T R. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 3.Johnson R, Lancki D V, Fitch F W. J Immunol. 1993;151:2986–2999. [PubMed] [Google Scholar]
- 4.Rock K L, Yeh E T H, Gramm C F, Haber S I, Reiser H, Benacerraf B. J Exp Med. 1986;163:315–333. doi: 10.1084/jem.163.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeClair K P, Bridgett M M, Dumont F J, Palfree R G E, Hämmerling U, Bothwell A L M. Eur J Immunol. 1989;19:1233–1239. doi: 10.1002/eji.1830190713. [DOI] [PubMed] [Google Scholar]
- 6.Herold K, Montag A G, Meyer S M, Wojcikowski C, Fitch F W. Diabetes. 1990;39:815–820. doi: 10.2337/diab.39.7.815. [DOI] [PubMed] [Google Scholar]
- 7.Jutila M A, Kroese F G M, Jutila K L, Stall A M, Fiering S, Herzenberg L A, Berg E L, Butcher E C. Eur J Immunol. 1988;18:1819–1862. doi: 10.1002/eji.1830181125. [DOI] [PubMed] [Google Scholar]
- 8.Yednock T A, Rosen S D. Adv Immunol. 1989;44:313–378. doi: 10.1016/s0065-2776(08)60645-8. [DOI] [PubMed] [Google Scholar]
- 9.Butcher E C. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 10.Vestweber D. Curr Top Microbiol Immunol. 1993;184:65–75. doi: 10.1007/978-3-642-78253-4_5. [DOI] [PubMed] [Google Scholar]
- 11.Holzmann B, Weissman I L. Cell. 1989;56:37–46. doi: 10.1016/0092-8674(89)90981-1. [DOI] [PubMed] [Google Scholar]
- 12.Hamann A, Jablonski-Westrich B, Duijvestin A, Butcher E C, Baisch H, Harder R, Thiele H G. J Immunol. 1988;140:693–699. [PubMed] [Google Scholar]
- 13.Berlin C, Bargatze R F, Campbell J J, von Andrian U H, Szabo M C, Hasslen S R, Nelson R D, Berg E L, Erlandsen S L, Butcher E C. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 14.Lo S K, Lee S, Ramos R A, Lobb R, Rosa M, Chi-Rosso G, Wright S D. J Exp Med. 1991;173:1493–1500. doi: 10.1084/jem.173.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Springer T A. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka Y, Adams D H, Shaw S. Immunol Today. 1993;14:111–115. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Adams D H, Hubscher S, Hirano H, Siebenlist U, Shaw S. Nature (London) 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 18.Mariani M, Camagna M, Tarditi L, Seccamani E. Mol Immunol. 1991;28:69–77. doi: 10.1016/0161-5890(91)90088-2. [DOI] [PubMed] [Google Scholar]
- 19.Hänninen A, Taylor C, Streeter P R, Stark L S, Sarte J M, Shizuru J A, Simell O, Michie S A. J Clin Invest. 1993;92:2509–2515. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamper H, Woodruff J. J Exp Med. 1976;144:828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jalkanen S T, Bargatze R F, de los Toyos J, Butcher E C. J Cell Biol. 1987;105:983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Madrid F, Simon P, Thompson S, Springer T A. J Exp Med. 1983;158:586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumont F J. J Immunol. 1987;138:4106–4113. [PubMed] [Google Scholar]
- 24.Philbrick W M, Maher S E, Bridgett M M, Bothwell A L M. EMBO J. 1990;9:2485–2492. doi: 10.1002/j.1460-2075.1990.tb07427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson P J. Immunol Today. 1991;12:35–41. doi: 10.1016/0167-5699(91)90110-F. [DOI] [PubMed] [Google Scholar]
- 26.Reiser H, Oettgen H, Yeh E T H, Terhorst C, Low M G, Benacerraf B, Rock K L. Cell. 1986;47:365–370. doi: 10.1016/0092-8674(86)90593-3. [DOI] [PubMed] [Google Scholar]
- 27.Malek T R, Ortega G, Chan C, Kroczek R, Shevach E M. J Exp Med. 1986;164:709–722. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh E T H, Reiser H, Daley J, Rock K L. J Immunol. 1987;138:91–97. [PubMed] [Google Scholar]
- 29.Havran W L, Lancki D W, Moldwin R L, Dialynas D P, Fitch F W. J Immunol. 1988;140:1034–1042. [PubMed] [Google Scholar]
- 30.Stefanova I, Horejsi V, Ansotegui I J, Knapp W, Stockinger H. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- 31.Bamezai A, Rock K L. Proc Natl Acad Sci USA. 1995;92:4294–4298. doi: 10.1073/pnas.92.10.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brakenhoff R H, Gerretsen M, Knippels E M C, van Dijk M, van Essen H, Weghuis D O, Sinke R J, Snow G B, van Dongen G A M S. J Cell Biol. 1995;129:1677–1689. doi: 10.1083/jcb.129.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koopman G, van Kooyk Y, de-Graaff M, Meyer C J, Figdor C G, Pals S T. J Immunol. 1990;145:3589–3593. [PubMed] [Google Scholar]
- 34.Isakov N, Mally M I, Scholz W, Altman A. Immunol Rev. 1987;95:89–111. doi: 10.1111/j.1600-065x.1987.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 35.Shattil S J, Brugge J S. Curr Opin Cell Biol. 1991;3:869–879. doi: 10.1016/0955-0674(91)90062-4. [DOI] [PubMed] [Google Scholar]