Abstract
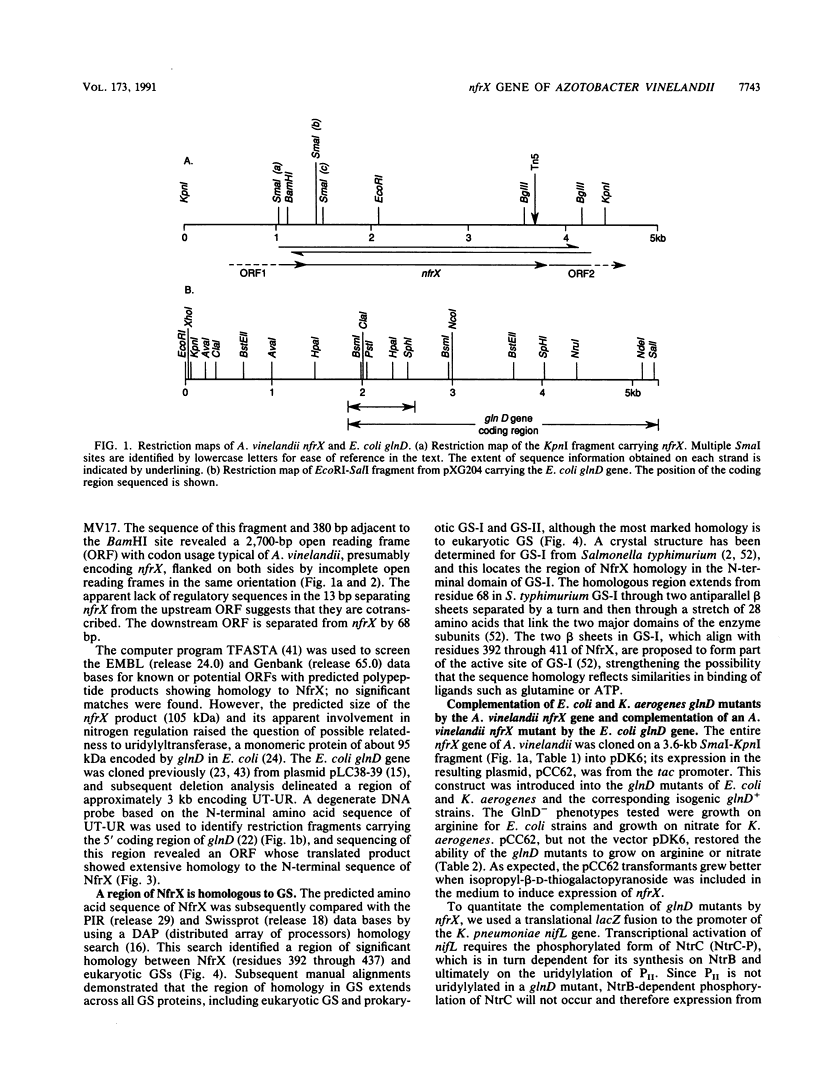
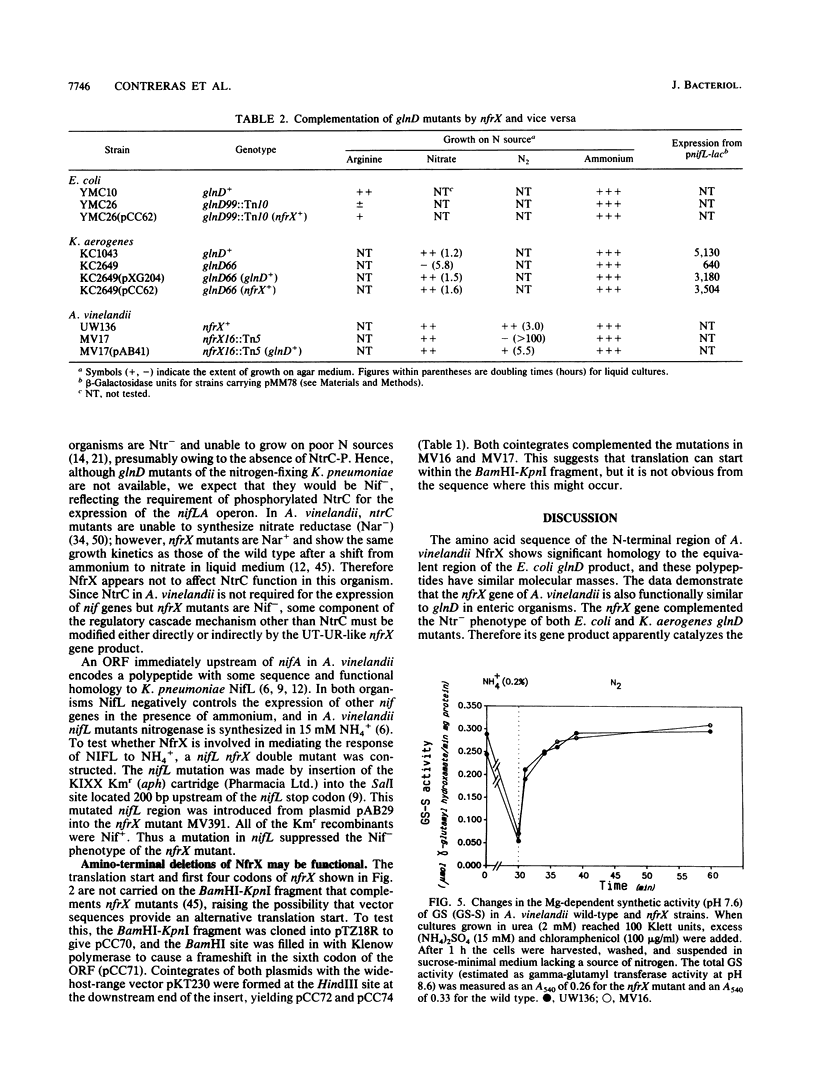
We sequenced the nitrogen fixation regulatory gene nfrX from Azotobacter vinelandii, mutations in which cause a Nif- phenotype, and found that it encodes a 105-kDa protein (NfrX), the N terminus of which is highly homologous to that of the uridylyltransferase-uridylyl-removing enzyme encoded by glnD in Escherichia coli. In vivo complementation experiments demonstrate that the glnD and nfrX products are functionally interchangeable. A vinelandii nfrX thus appears to encode a uridylyltransferase-uridylyl-removing enzyme, and in this paper we report the first sequence of such a protein. The Nif- phenotype of nfrX mutants can be suppressed by a second mutation in a recently identified nifL-like gene immediately upstream of nifA in A. vinelandii. NifL mediates nif regulation in response to the N status in A. vinelandii, presumably by inhibiting NifA activator function as occurs in Klebsiella pneumoniae; thus, one role of NfrX is to modify, either directly or indirectly, the activity of the nifL product.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. P., Purich D., Stadtman E. R. Cascade control of Escherichia coli glutamine synthetase. Properties of the PII regulatory protein and the uridylyltransferase-uridylyl-removing enzyme. J Biol Chem. 1975 Aug 25;250(16):6264–6272. [PubMed] [Google Scholar]
- Almassy R. J., Janson C. A., Hamlin R., Xuong N. H., Eisenberg D. Novel subunit-subunit interactions in the structure of glutamine synthetase. 1986 Sep 25-Oct 1Nature. 323(6086):304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- Austin S., Henderson N., Dixon R. Requirements for transcriptional activation in vitro of the nitrogen-regulated glnA and nifLA promoters from Klebsiella pneumoniae: dependence on activator concentration. Mol Microbiol. 1987 Jul;1(1):92–100. doi: 10.1111/j.1365-2958.1987.tb00532.x. [DOI] [PubMed] [Google Scholar]
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Bancroft S., Rhee S. G., Neumann C., Kustu S. Mutations that alter the covalent modification of glutamine synthetase in Salmonella typhimurium. J Bacteriol. 1978 Jun;134(3):1046–1055. doi: 10.1128/jb.134.3.1046-1055.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett L. T., Cannon F., Dean D. R. Nucleotide sequence and mutagenesis of the nifA gene from Azotobacter vinelandii. Mol Microbiol. 1988 May;2(3):315–321. doi: 10.1111/j.1365-2958.1988.tb00034.x. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Brill W. J. Genetic analysis of Azotobacter vinelandii mutant strains unable to fix nitrogen. J Bacteriol. 1977 May;130(2):954–956. doi: 10.1128/jb.130.2.954-956.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom F. R., Levin M. S., Foor F., Tyler B. Regulation of glutamine synthetase formation in Escherichia coli: characterization of mutants lacking the uridylyltransferase. J Bacteriol. 1978 May;134(2):569–577. doi: 10.1128/jb.134.2.569-577.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno R., Pahel G., Magasanik B. Role of glnB and glnD gene products in regulation of the glnALG operon of Escherichia coli. J Bacteriol. 1985 Nov;164(2):816–822. doi: 10.1128/jb.164.2.816-822.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Collins J. F., Coulson A. F., Lyall A. The significance of protein sequence similarities. Comput Appl Biosci. 1988 Mar;4(1):67–71. doi: 10.1093/bioinformatics/4.1.67. [DOI] [PubMed] [Google Scholar]
- Davison J., Heusterspreute M., Chevalier N., Ha-Thi V., Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51(2-3):275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- Dixon R., Kennedy C., Kondorosi A., Krishnapillai V., Merrick M. Complementation analysis of Klebsiella pneumoniae mutants defective in nitrogen fixation. Mol Gen Genet. 1977 Nov 29;157(2):189–198. doi: 10.1007/BF00267397. [DOI] [PubMed] [Google Scholar]
- Drummond M., Whitty P., Wootton J. Sequence and domain relationships of ntrC and nifA from Klebsiella pneumoniae: homologies to other regulatory proteins. EMBO J. 1986 Feb;5(2):441–447. doi: 10.1002/j.1460-2075.1986.tb04230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foor F., Cedergren R. J., Streicher S. L., Rhee S. G., Magasanik B. Glutamine synthetase of Klebsiella aerogenes: properties of glnD mutants lacking uridylyltransferase. J Bacteriol. 1978 May;134(2):562–568. doi: 10.1128/jb.134.2.562-568.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia E., Federici M., Rhee S. G., Berberich M. A. Glutamine synthetase cascade: enrichment of uridylyltransferase in Escherichia coli carrying hybrid ColE1 plasmids. Arch Biochem Biophys. 1980 Aug;203(1):181–189. doi: 10.1016/0003-9861(80)90167-8. [DOI] [PubMed] [Google Scholar]
- Garcia E., Rhee S. G. Cascade control of Escherichia coli glutamine synthetase. Purification and properties of PII uridylyltransferase and uridylyl-removing enzyme. J Biol Chem. 1983 Feb 25;258(4):2246–2253. [PubMed] [Google Scholar]
- Holtel A., Merrick M. J. The Klebsiella pneumoniae PII protein (glnB gene product) is not absolutely required for nitrogen regulation and is not involved in NifL-mediated nif gene regulation. Mol Gen Genet. 1989 Jun;217(2-3):474–480. doi: 10.1007/BF02464920. [DOI] [PubMed] [Google Scholar]
- Keener J., Kustu S. Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4976–4980. doi: 10.1073/pnas.85.14.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C. Linkage map of the nitrogen fixation (nif) genes in Klebsiella pneumoniae. Mol Gen Genet. 1977 Nov 29;157(2):199–204. doi: 10.1007/BF00267398. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Toukdarian A. Genetics of azotobacters: applications to nitrogen fixation and related aspects of metabolism. Annu Rev Microbiol. 1987;41:227–258. doi: 10.1146/annurev.mi.41.100187.001303. [DOI] [PubMed] [Google Scholar]
- Kleiner D., Paul W., Merrick M. J. Construction of multicopy expression vectors for regulated over-production of proteins in Klebsiella pneumoniae and other enteric bacteria. J Gen Microbiol. 1988 Jul;134(7):1779–1784. doi: 10.1099/00221287-134-7-1779. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Kleiner D. The glutamine synthetase from Azotobacter vinelandii: purification, characterization, regulation and localization. Eur J Biochem. 1978 Aug 15;89(1):51–60. doi: 10.1111/j.1432-1033.1978.tb20895.x. [DOI] [PubMed] [Google Scholar]
- Macaluso A., Best E. A., Bender R. A. Role of the nac gene product in the nitrogen regulation of some NTR-regulated operons of Klebsiella aerogenes. J Bacteriol. 1990 Dec;172(12):7249–7255. doi: 10.1128/jb.172.12.7249-7255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON J. W., WILSON P. W., BURRIS R. H. Direct demonstration of ammonia as an intermediate in nitrogen fixation by Azotobacter. J Biol Chem. 1953 Sep;204(1):445–451. [PubMed] [Google Scholar]
- Ninfa A. J., Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill E. A., Kiely G. M., Bender R. A. Transposon Tn5 encodes streptomycin resistance in nonenteric bacteria. J Bacteriol. 1984 Jul;159(1):388–389. doi: 10.1128/jb.159.1.388-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. G., Park S. C., Koo J. H. The role of adenylyltransferase and uridylyltransferase in the regulation of glutamine synthetase in Escherichia coli. Curr Top Cell Regul. 1985;27:221–232. doi: 10.1016/b978-0-12-152827-0.50026-8. [DOI] [PubMed] [Google Scholar]
- Richaud C., Richaud F., Martin C., Haziza C., Patte J. C. Regulation of expression and nucleotide sequence of the Escherichia coli dapD gene. J Biol Chem. 1984 Dec 10;259(23):14824–14828. [PubMed] [Google Scholar]
- Robson R. L., Chesshyre J. A., Wheeler C., Jones R., Woodley P. R., Postgate J. R. Genome size and complexity in Azotobacter chroococcum. J Gen Microbiol. 1984 Jul;130(7):1603–1612. doi: 10.1099/00221287-130-7-1603. [DOI] [PubMed] [Google Scholar]
- Santero E., Toukdarian A., Humphrey R., Kennedy C. Identification and characterization of two nitrogen fixation regulatory regions, nifA and nfrX, in Azotobacter vinelandii and Azotobacter chroococcum. Mol Microbiol. 1988 May;2(3):303–314. doi: 10.1111/j.1365-2958.1988.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Shyamala V., Ames G. F. Amplification of bacterial genomic DNA by the polymerase chain reaction and direct sequencing after asymmetric amplification: application to the study of periplasmic permeases. J Bacteriol. 1989 Mar;171(3):1602–1608. doi: 10.1128/jb.171.3.1602-1608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedel J., Shelton E. Purification and properties of Azotobacter vinelandii glutamine synthetase. Arch Biochem Biophys. 1979 Jan;192(1):214–224. doi: 10.1016/0003-9861(79)90086-9. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toukdarian A., Kennedy C. Regulation of nitrogen metabolism in Azotobacter vinelandii: isolation of ntr and glnA genes and construction of ntr mutants. EMBO J. 1986 Feb;5(2):399–407. doi: 10.1002/j.1460-2075.1986.tb04225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toukdarian A., Saunders G., Selman-Sosa G., Santero E., Woodley P., Kennedy C. Molecular analysis of the Azotobacter vinelandii glnA gene encoding glutamine synthetase. J Bacteriol. 1990 Nov;172(11):6529–6539. doi: 10.1128/jb.172.11.6529-6539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M. M., Almassy R. J., Janson C. A., Cascio D., Eisenberg D. Refined atomic model of glutamine synthetase at 3.5 A resolution. J Biol Chem. 1989 Oct 25;264(30):17681–17690. doi: 10.2210/pdb2gls/pdb. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



