Abstract
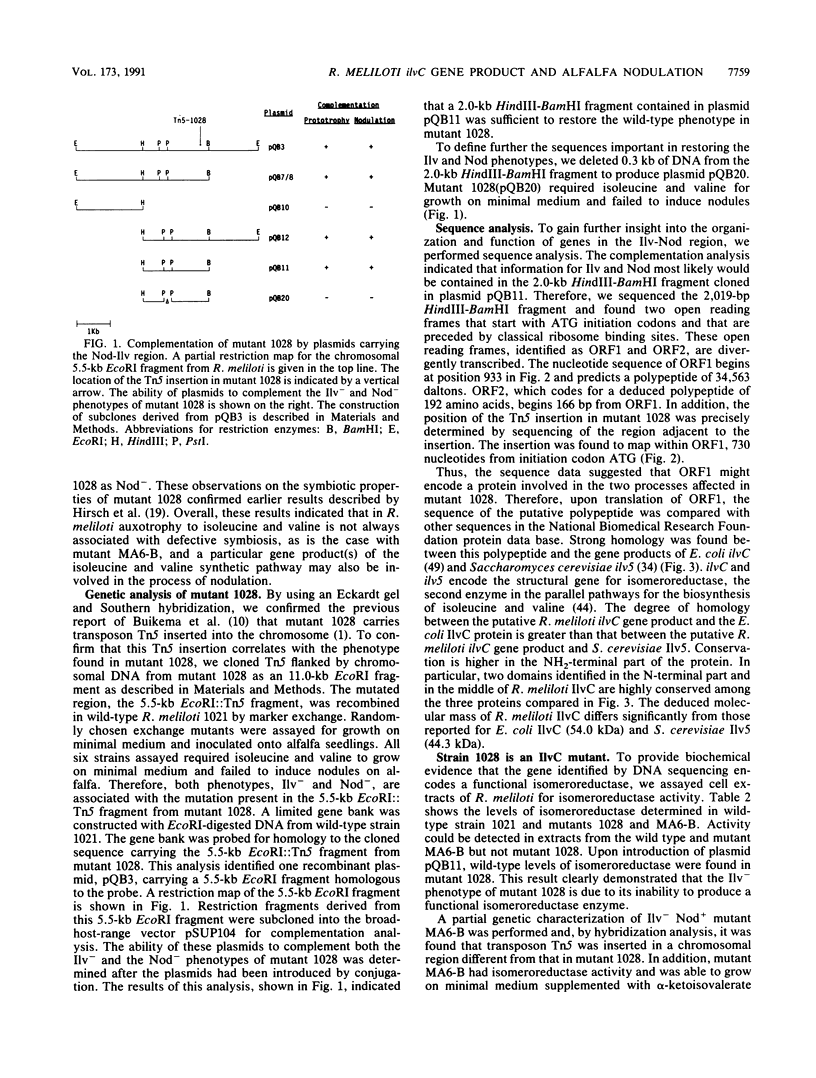
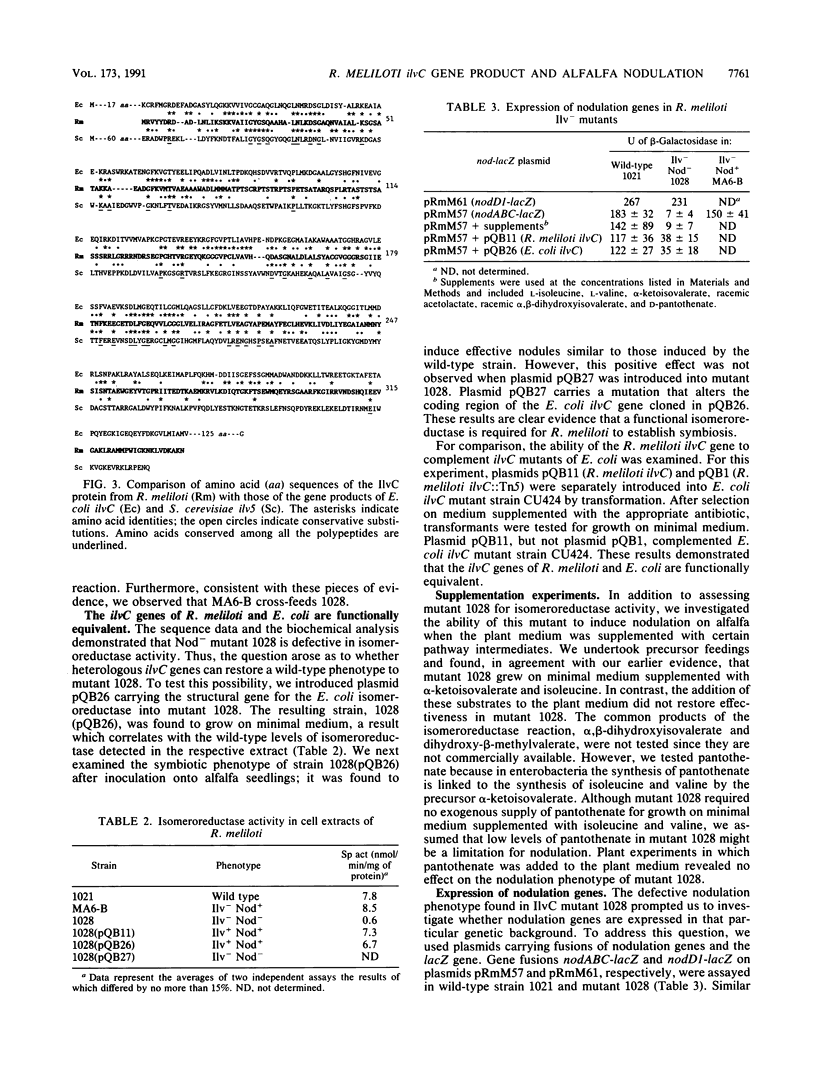
Tn5-induced mutants of Rhizobium meliloti that require the amino acids isoleucine and valine for growth on minimal medium were studied. In one mutant, 1028, the defect is associated with an inability to induce nodules on alfalfa. The Tn5 mutation in 1028 is located in a chromosomal 5.5-kb EcoRI fragment. Complementation analysis with cloned DNA indicated that 2.0 kb of DNA from the 5.5-kb EcoRI fragment restored the wild-type phenotype in the Ilv- Nod- mutant. This region was further characterized by DNA sequence analysis and was shown to contain a coding sequence homologous to those for Escherichia coli IlvC and Saccharomyces cerevisiae Ilv5. Genes ilvC and ilv5 code for the enzyme acetohydroxy acid isomeroreductase (isomeroreductase), the second enzyme in the parallel pathways for the biosynthesis of isoleucine and valine. Enzymatic assays confirmed that strain 1028 was a mutant defective in isomeroreductase activity. In addition, it was shown that the ilvC genes of Rhizobium meliloti and E. coli are functionally equivalent. We demonstrated that in ilvC mutant 1028 the common nodulation genes nodABC are not activated by the inducer luteolin. E. coli ilvC complemented both defective properties (Ilv- and Nod-) found in mutant 1028. These findings demonstrate that R. meliloti requires an active isomeroreductase enzyme for successful nodulation of alfalfa.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar O. M., Kapp D., Pühler A. Characterization of a Rhizobium meliloti fixation gene (fixF) located near the common nodulation region. J Bacteriol. 1985 Oct;164(1):245–254. doi: 10.1128/jb.164.1.245-254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar O. M., Taormino J., Thöny B., Ramseier T., Hennecke H., Szalay A. A. The nifEN genes participating in FeMo cofactor biosynthesis and genes encoding dinitrogenase are part of the same operon in Bradyrhizobium species. Mol Gen Genet. 1990 Dec;224(3):413–420. doi: 10.1007/BF00262436. [DOI] [PubMed] [Google Scholar]
- Arfin S. M., Ratzkin B., Umbarger H. E. The metabolism of valine and isoleucine in Escherichia coli. XVII. The role of induction in the derepression of acetohydroxy acid isomeroreductase. Biochem Biophys Res Commun. 1969 Dec 4;37(6):902–908. doi: 10.1016/0006-291x(69)90216-2. [DOI] [PubMed] [Google Scholar]
- Aulabaugh A., Schloss J. V. Oxalyl hydroxamates as reaction-intermediate analogues for ketol-acid reductoisomerase. Biochemistry. 1990 Mar 20;29(11):2824–2830. doi: 10.1021/bi00463a027. [DOI] [PubMed] [Google Scholar]
- Blazey D. L., Burns R. O. Regulation of Salmonella typhimurium ilvYC genes. J Bacteriol. 1984 Sep;159(3):951–957. doi: 10.1128/jb.159.3.951-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Buikema W. J., Long S. R., Brown S. E., van den Bos R. C., Earl C., Ausubel F. M. Physical and genetic characterization of Rhizobium meliloti symbiotic mutants. J Mol Appl Genet. 1983;2(3):249–260. [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debellé F., Rosenberg C., Vasse J., Maillet F., Martinez E., Dénarié J., Truchet G. Assignment of symbiotic developmental phenotypes to common and specific nodulation (nod) genetic loci of Rhizobium meliloti. J Bacteriol. 1986 Dec;168(3):1075–1086. doi: 10.1128/jb.168.3.1075-1086.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debellé F., Sharma S. B. Nucleotide sequence of Rhizobium meliloti RCR2011 genes involved in host specificity of nodulation. Nucleic Acids Res. 1986 Sep 25;14(18):7453–7472. doi: 10.1093/nar/14.18.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelke T., Jording D., Kapp D., Pühler A. Identification and sequence analysis of the Rhizobium meliloti dctA gene encoding the C4-dicarboxylate carrier. J Bacteriol. 1989 Oct;171(10):5551–5560. doi: 10.1128/jb.171.10.5551-5560.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucher C., Maillet F., Vasse J., Rosenberg C., van Brussel A. A., Truchet G., Dénarié J. Rhizobium meliloti host range nodH gene determines production of an alfalfa-specific extracellular signal. J Bacteriol. 1988 Dec;170(12):5489–5499. doi: 10.1128/jb.170.12.5489-5499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger O., Spaink H. P., Kennedy E. P. Isolation of the Rhizobium leguminosarum NodF nodulation protein: NodF carries a 4'-phosphopantetheine prosthetic group. J Bacteriol. 1991 May;173(9):2872–2878. doi: 10.1128/jb.173.9.2872-2878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia R. A., Cavaignac S., Zorreguieta A., Toro N., Olivares J., Ugalde R. A. A Rhizobium meliloti mutant that forms ineffective pseudonodules in alfalfa produces exopolysaccharide but fails to form beta-(1----2) glucan. J Bacteriol. 1987 Feb;169(2):880–884. doi: 10.1128/jb.169.2.880-884.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Long S. R., Bang M., Haskins N., Ausubel F. M. Structural studies of alfalfa roots infected with nodulation mutants of Rhizobium meliloti. J Bacteriol. 1982 Jul;151(1):411–419. doi: 10.1128/jb.151.1.411-419.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma M. A., Asomaning M., Ausubel F. M. Rhizobium meliloti nodD genes mediate host-specific activation of nodABC. J Bacteriol. 1990 Feb;172(2):901–911. doi: 10.1128/jb.172.2.901-911.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B., Kondorosi E., John M., Schmidt J., Török I., Györgypal Z., Barabas I., Wieneke U., Schell J., Kondorosi A. Organization, structure and symbiotic function of Rhizobium meliloti nodulation genes determining host specificity for alfalfa. Cell. 1986 Aug 1;46(3):335–343. doi: 10.1016/0092-8674(86)90654-9. [DOI] [PubMed] [Google Scholar]
- Leigh J. A., Lee C. C. Characterization of polysaccharides of Rhizobium meliloti exo mutants that form ineffective nodules. J Bacteriol. 1988 Aug;170(8):3327–3332. doi: 10.1128/jb.170.8.3327-3332.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Peters N. K., Long S. R. Alfalfa Root Exudates and Compounds which Promote or Inhibit Induction of Rhizobium meliloti Nodulation Genes. Plant Physiol. 1988 Oct;88(2):396–400. doi: 10.1104/pp.88.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J. G., Holmberg S. The ILV5 gene of Saccharomyces cerevisiae is highly expressed. Nucleic Acids Res. 1986 Dec 22;14(24):9631–9651. doi: 10.1093/nar/14.24.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primerano D. A., Burns R. O. Role of acetohydroxy acid isomeroreductase in biosynthesis of pantothenic acid in Salmonella typhimurium. J Bacteriol. 1983 Jan;153(1):259–269. doi: 10.1128/jb.153.1.259-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi V. K., Watson R. J. Aspartate aminotransferase activity is required for aspartate catabolism and symbiotic nitrogen fixation in Rhizobium meliloti. J Bacteriol. 1991 May;173(9):2879–2887. doi: 10.1128/jb.173.9.2879-2887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Schwedock J., Long S. R. ATP sulphurylase activity of the nodP and nodQ gene products of Rhizobium meliloti. Nature. 1990 Dec 13;348(6302):644–647. doi: 10.1038/348644a0. [DOI] [PubMed] [Google Scholar]
- Shearman C. A., Rossen L., Johnston A. W., Downie J. A. The Rhizobium leguminosarum nodulation gene nodF encodes a polypeptide similar to acyl-carrier protein and is regulated by nodD plus a factor in pea root exudate. EMBO J. 1986 Apr;5(4):647–652. doi: 10.1002/j.1460-2075.1986.tb04262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield S. W., Ielpi L., O'Brochta D., Helinski D. R., Ditta G. S. The ndvA gene product of Rhizobium meliloti is required for beta-(1----2)glucan production and has homology to the ATP-binding export protein HlyB. J Bacteriol. 1988 Aug;170(8):3523–3530. doi: 10.1128/jb.170.8.3523-3530.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Renz M. An optimized freeze-squeeze method for the recovery of DNA fragments from agarose gels. Anal Biochem. 1983 Jul 1;132(1):14–19. doi: 10.1016/0003-2697(83)90419-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Watson M. D., Wild J., Umbarger H. E. Positive control of ilvC expression in Escherichia coli K-12; identification and mapping of regulatory gene ilvY. J Bacteriol. 1979 Sep;139(3):1014–1020. doi: 10.1128/jb.139.3.1014-1020.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Chan Y. K., Wheatcroft R., Yang A. F., Han S. H. Rhizobium meliloti genes required for C4-dicarboxylate transport and symbiotic nitrogen fixation are located on a megaplasmid. J Bacteriol. 1988 Feb;170(2):927–934. doi: 10.1128/jb.170.2.927-934.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Hatfield G. W. Nucleotide sequence and in vivo expression of the ilvY and ilvC genes in Escherichia coli K12. Transcription from divergent overlapping promoters. J Biol Chem. 1986 Feb 15;261(5):2441–2450. [PubMed] [Google Scholar]



