Abstract
In the course of developing strategies to obtain a mutation in the aspartate semialdehyde dehydrogenase (asd) gene of Mycobacterium smegmatis, an efficient transposon trap was constructed which may be generally useful for the identification of transposable elements in mycobacteria. A DNA fragment containing the asd gene was replaced with an aminoglycoside phosphotransferase gene (aph) to generate a delta asd::aph allele. Attempts to replace the wild-type asd gene with the delta asd::aph allele were unsuccessful, suggesting that this deletion was lethal to the growth of M. smegmatis. The plasmid, pYUB215, which contains beta-galactosidase expressed from a mycobacteriophage promoter and delta asd::aph, was integrated into the chromosome of M. smegmatis by a homologous, single-crossover, recombination event. Visual screening for inactivation of the beta-galactosidase gene in the resulting strain allowed the isolation of a novel mycobacterial insertion element from M. smegmatis. This insertion element, which is unique to M. smegmatis, was designated IS1096 and transposes at a frequency of 7.2 x 10(-5) per cell in an apparently random fashion. IS1096 is 2,275 bp in length and contains two open reading frames which are predicted to encode proteins involved in transposition. This insertion element exhibits several characteristics that suggest it may be a useful tool for genetic analysis of mycobacteria, possibly including the study of mechanisms of pathogenesis.
Full text
PDF


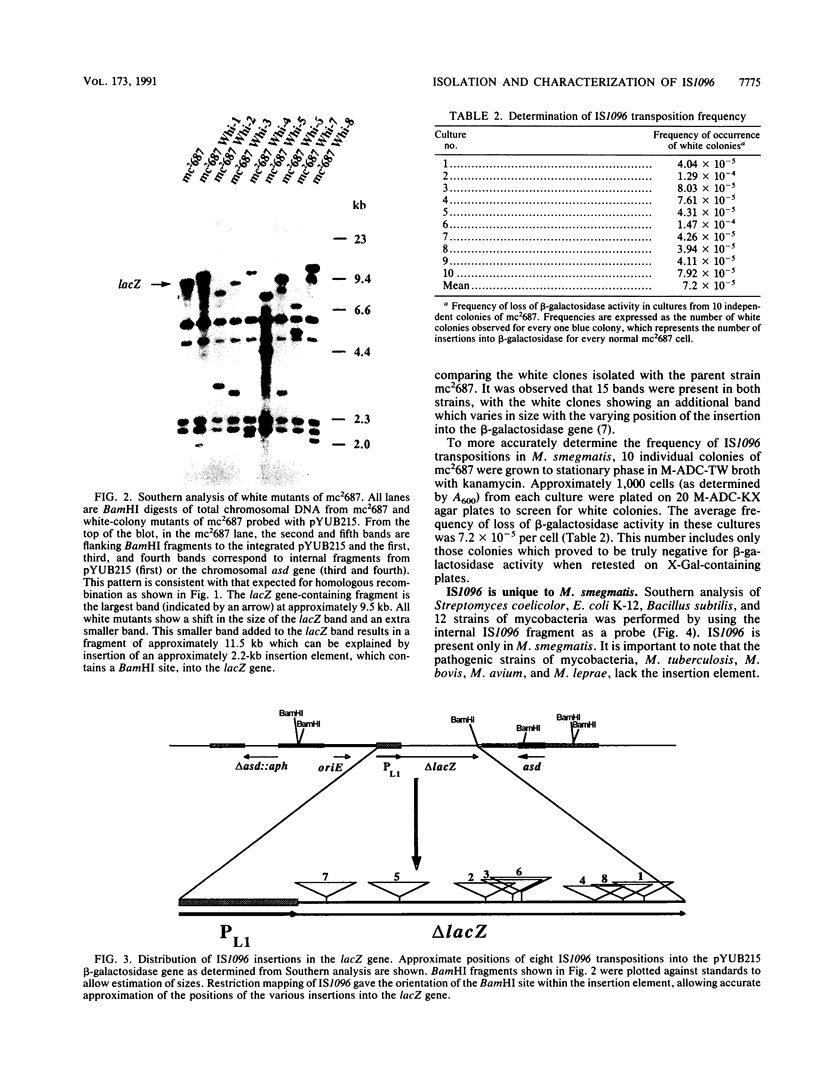





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldovini A., Young R. A. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature. 1991 Jun 6;351(6326):479–482. doi: 10.1038/351479a0. [DOI] [PubMed] [Google Scholar]
- Belisle J. T., Pascopella L., Inamine J. M., Brennan P. J., Jacobs W. R., Jr Isolation and expression of a gene cluster responsible for biosynthesis of the glycopeptidolipid antigens of Mycobacterium avium. J Bacteriol. 1991 Nov;173(21):6991–6997. doi: 10.1128/jb.173.21.6991-6997.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach K. D., Cave M. D., Bates J. H., Crawford J. T. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis. 1990 May;161(5):977–981. doi: 10.1093/infdis/161.5.977. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Fisher S. H., Bruton C. J., Chater K. F. The glucose kinase gene of Streptomyces coelicolor and its use in selecting spontaneous deletions for desired regions of the genome. Mol Gen Genet. 1987 Jan;206(1):35–44. doi: 10.1007/BF00326533. [DOI] [PubMed] [Google Scholar]
- Green E. P., Tizard M. L., Moss M. T., Thompson J., Winterbourne D. J., McFadden J. J., Hermon-Taylor J. Sequence and characteristics of IS900, an insertion element identified in a human Crohn's disease isolate of Mycobacterium paratuberculosis. Nucleic Acids Res. 1989 Nov 25;17(22):9063–9073. doi: 10.1093/nar/17.22.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskinsky C. M., Jacobs W. R., Jr, Clark-Curtiss J. E., Bloom B. R. Genetic relationships among Mycobacterium leprae, Mycobacterium tuberculosis, and candidate leprosy vaccine strains determined by DNA hybridization: identification of an M. leprae-specific repetitive sequence. Infect Immun. 1989 May;57(5):1535–1541. doi: 10.1128/iai.57.5.1535-1541.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson R. N., James B. E., Young R. A. Gene replacement and expression of foreign DNA in mycobacteria. J Bacteriol. 1990 Feb;172(2):519–524. doi: 10.1128/jb.172.2.519-524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. Genetic analysis of bacterial virulence determinants in Bordetella pertussis and the pathogenetic Yersinia. Curr Top Microbiol Immunol. 1985;118:1–11. doi: 10.1007/978-3-642-70586-1_1. [DOI] [PubMed] [Google Scholar]
- Jacobs W. R., Jr, Kalpana G. V., Cirillo J. D., Pascopella L., Snapper S. B., Udani R. A., Jones W., Barletta R. G., Bloom B. R. Genetic systems for mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- Jacobs W. R., Jr, Tuckman M., Bloom B. R. Introduction of foreign DNA into mycobacteria using a shuttle phasmid. Nature. 1987 Jun 11;327(6122):532–535. doi: 10.1038/327532a0. [DOI] [PubMed] [Google Scholar]
- Kalpana G. V., Bloom B. R., Jacobs W. R., Jr Insertional mutagenesis and illegitimate recombination in mycobacteria. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5433–5437. doi: 10.1073/pnas.88.12.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Lee M. H., Pascopella L., Jacobs W. R., Jr, Hatfull G. F. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and bacille Calmette-Guérin. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3111–3115. doi: 10.1073/pnas.88.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Timm J., Rauzier J., Gomez-Lus R., Davies J., Gicquel B. Transposition of an antibiotic resistance element in mycobacteria. Nature. 1990 Jun 21;345(6277):739–743. doi: 10.1038/345739a0. [DOI] [PubMed] [Google Scholar]
- Martinez E., de la Cruz F. Transposon Tn21 encodes a RecA-independent site-specific integration system. Mol Gen Genet. 1988 Feb;211(2):320–325. doi: 10.1007/BF00330610. [DOI] [PubMed] [Google Scholar]
- McAdam R. A., Hermans P. W., van Soolingen D., Zainuddin Z. F., Catty D., van Embden J. D., Dale J. W. Characterization of a Mycobacterium tuberculosis insertion sequence belonging to the IS3 family. Mol Microbiol. 1990 Sep;4(9):1607–1613. doi: 10.1111/j.1365-2958.1990.tb02073.x. [DOI] [PubMed] [Google Scholar]
- McFadden J. J., Butcher P. D., Chiodini R., Hermon-Taylor J. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis, as determined by DNA probes that distinguish between mycobacterial species. J Clin Microbiol. 1987 May;25(5):796–801. doi: 10.1128/jcm.25.5.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M L, Lederberg E M, Lederberg J. Transductional Heterogenotes in Escherichia Coli. Genetics. 1956 Sep;41(5):758–779. doi: 10.1093/genetics/41.5.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. J., Fries J. W., Piessens W. F., Wirth D. F. Sequence analysis and amplification by polymerase chain reaction of a cloned DNA fragment for identification of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Mar;28(3):513–518. doi: 10.1128/jcm.28.3.513-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranes M. G., Rauzier J., Lagranderie M., Gheorghiu M., Gicquel B. Functional analysis of pAL5000, a plasmid from Mycobacterium fortuitum: construction of a "mini" mycobacterium-Escherichia coli shuttle vector. J Bacteriol. 1990 May;172(5):2793–2797. doi: 10.1128/jb.172.5.2793-2797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. R., Shibuya G. I., Steitz J. A. Nucleotide sequence of gamma delta resolvase gene and demonstration that its gene product acts as a repressor of transcription. Nature. 1982 Nov 25;300(5890):381–383. doi: 10.1038/300381a0. [DOI] [PubMed] [Google Scholar]
- Rowland S. J., Dyke K. G. Tn552, a novel transposable element from Staphylococcus aureus. Mol Microbiol. 1990 Jun;4(6):961–975. doi: 10.1111/j.1365-2958.1990.tb00669.x. [DOI] [PubMed] [Google Scholar]
- Segall A. M., Roth J. R. Recombination between homologies in direct and inverse orientation in the chromosome of Salmonella: intervals which are nonpermissive for inversion formation. Genetics. 1989 Aug;122(4):737–747. doi: 10.1093/genetics/122.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S. B., Lugosi L., Jekkel A., Melton R. E., Kieser T., Bloom B. R., Jacobs W. R., Jr Lysogeny and transformation in mycobacteria: stable expression of foreign genes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6987–6991. doi: 10.1073/pnas.85.18.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S. B., Melton R. E., Mustafa S., Kieser T., Jacobs W. R., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990 Nov;4(11):1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stover C. K., de la Cruz V. F., Fuerst T. R., Burlein J. E., Benson L. A., Bennett L. T., Bansal G. P., Young J. F., Lee M. H., Hatfull G. F. New use of BCG for recombinant vaccines. Nature. 1991 Jun 6;351(6326):456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Manoil C., Mekalanos J. J. Broad-host-range vectors for delivery of TnphoA: use in genetic analysis of secreted virulence determinants of Vibrio cholerae. J Bacteriol. 1989 Apr;171(4):1870–1878. doi: 10.1128/jb.171.4.1870-1878.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaraj H. S., Lamb F. I., Davis E. O., Jenner P. J., Jeyakumar L. H., Colston M. J. Identification, sequencing, and expression of Mycobacterium leprae superoxide dismutase, a major antigen. Infect Immun. 1990 Jun;58(6):1937–1942. doi: 10.1128/iai.58.6.1937-1942.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry D., Brisson-Noël A., Vincent-Lévy-Frébault V., Nguyen S., Guesdon J. L., Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol. 1990 Dec;28(12):2668–2673. doi: 10.1128/jcm.28.12.2668-2673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry D., Cave M. D., Eisenach K. D., Crawford J. T., Bates J. H., Gicquel B., Guesdon J. L. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 1990 Jan 11;18(1):188–188. doi: 10.1093/nar/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. K., Grinsted J. DNA sequence of the transposase gene of the class II transposon, Tn3926. Nucleic Acids Res. 1989 Feb 25;17(4):1757–1757. doi: 10.1093/nar/17.4.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Wayne L. G., Gross W. M. Base composition of deoxyribonucleic acid isolated from mycobacteria. J Bacteriol. 1968 Dec;96(6):1915–1919. doi: 10.1128/jb.96.6.1915-1919.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh P., Sicard A. M., Sinskey A. J. General organization of the genes specifically involved in the diaminopimelate-lysine biosynthetic pathway of Corynebacterium glutamicum. Mol Gen Genet. 1988 Apr;212(1):105–111. doi: 10.1007/BF00322451. [DOI] [PubMed] [Google Scholar]






