Abstract
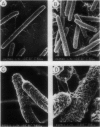
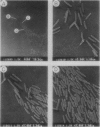
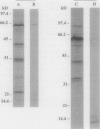
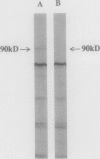
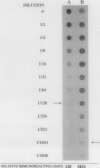
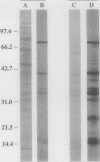
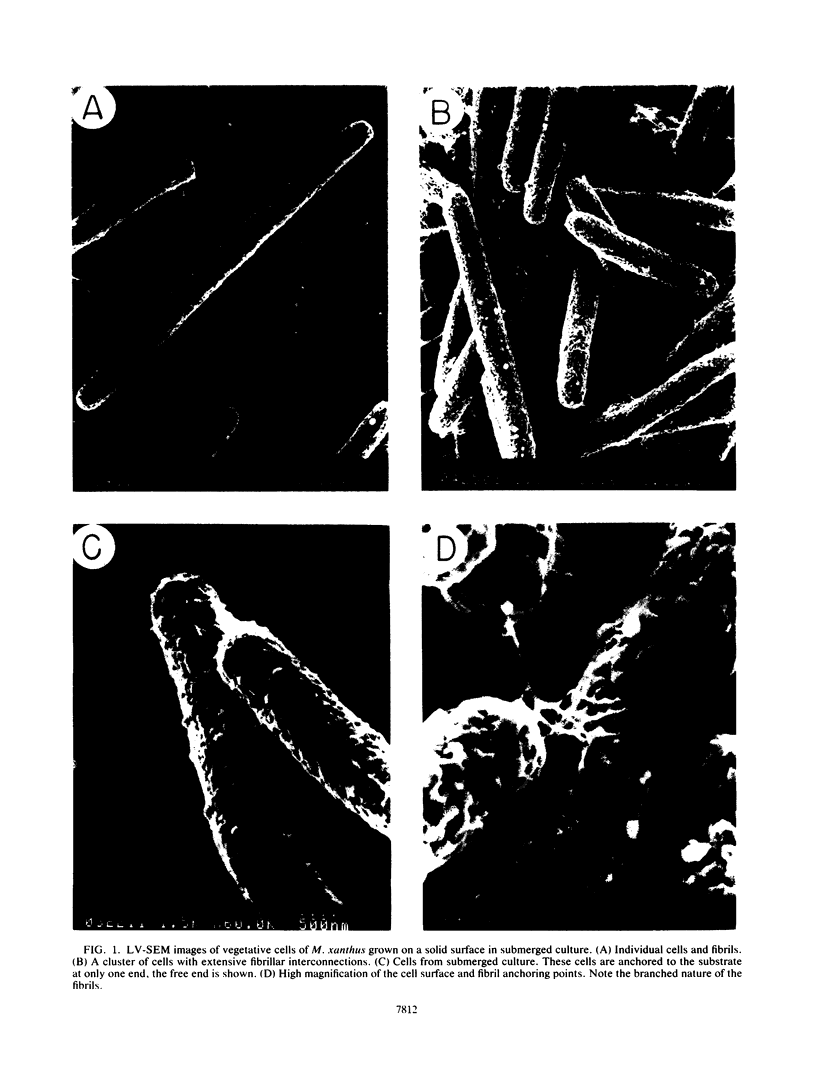
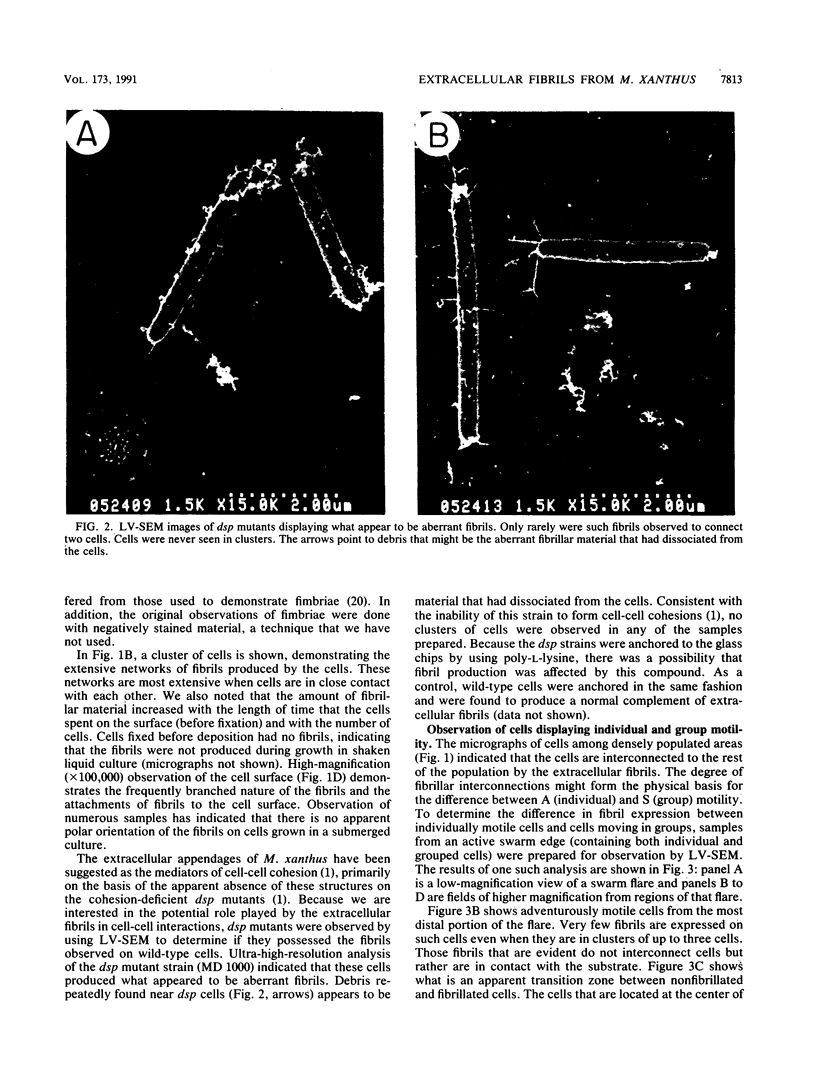
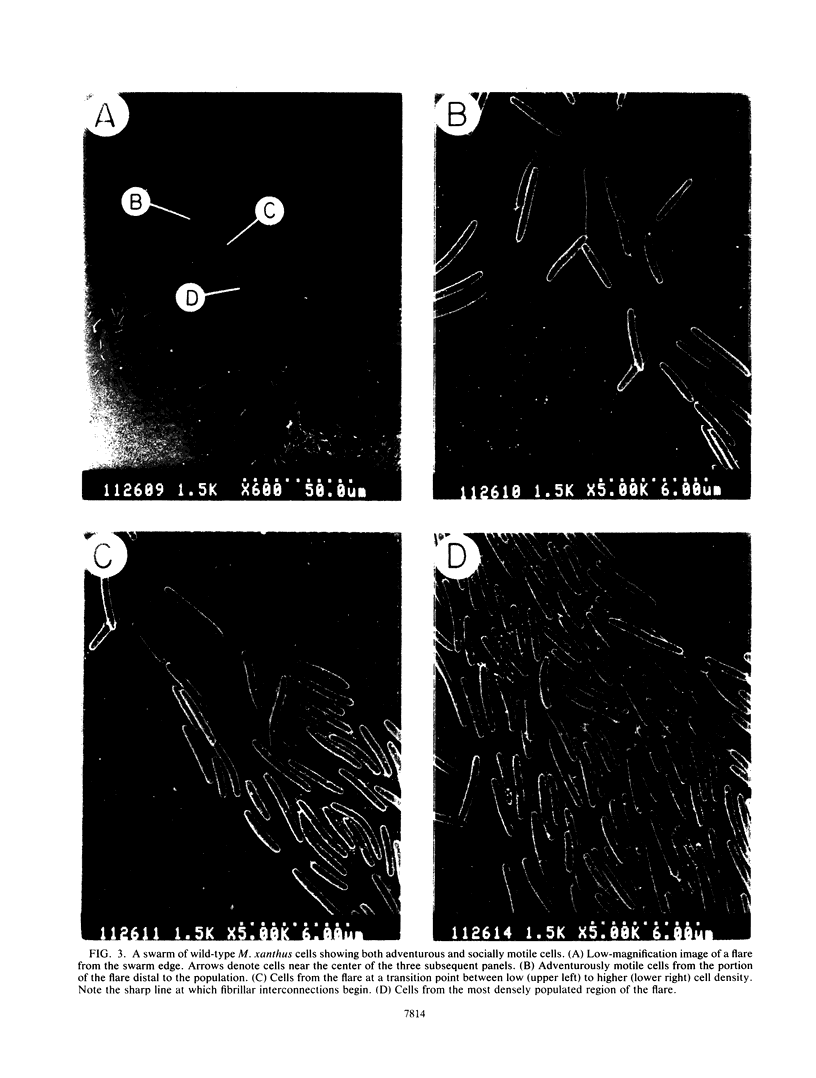
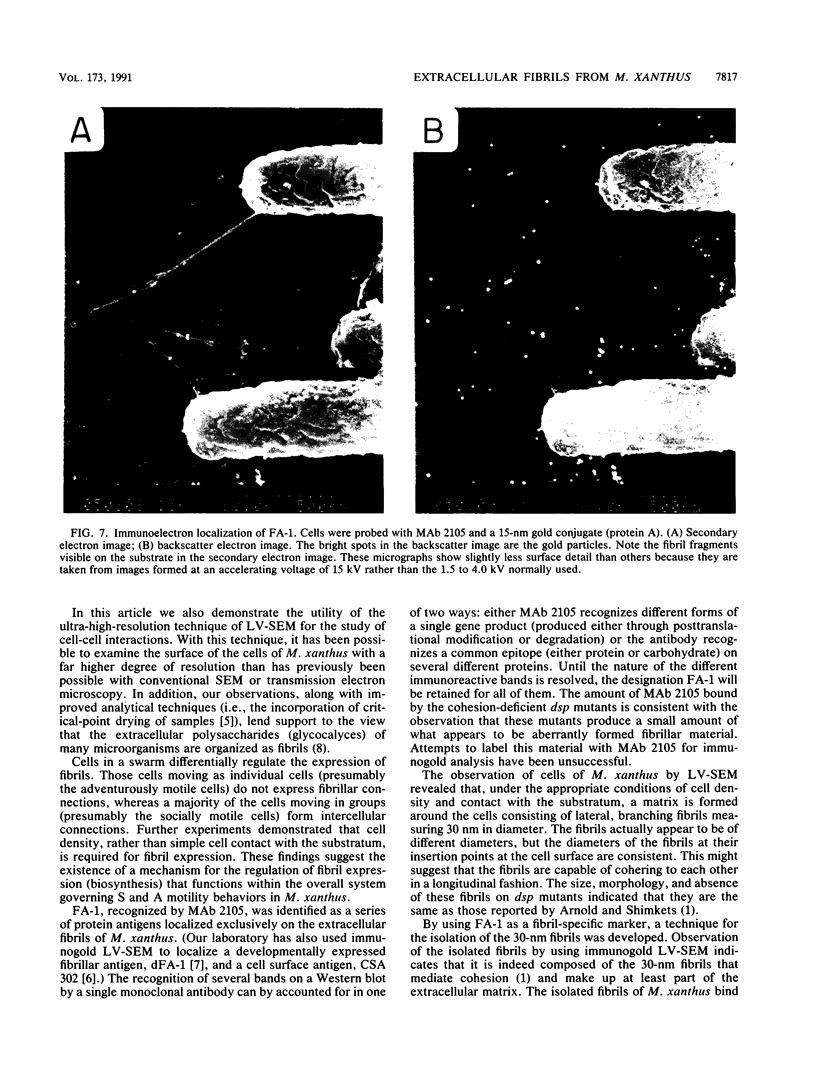
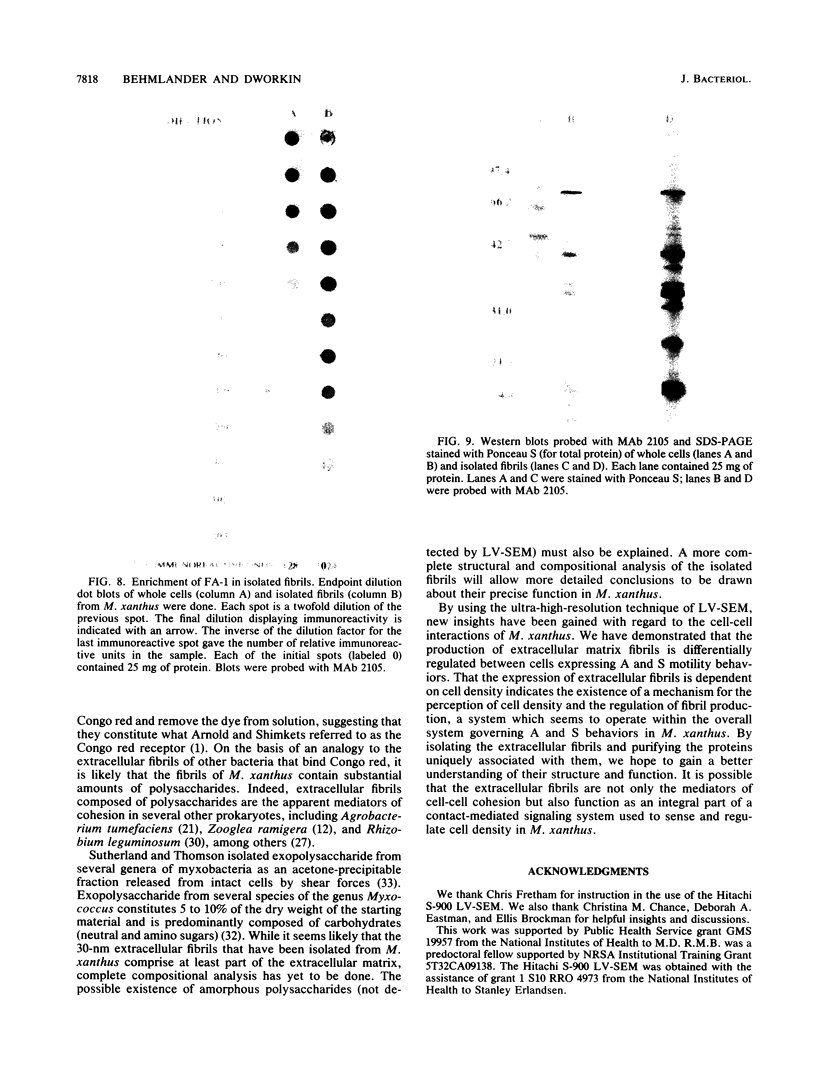
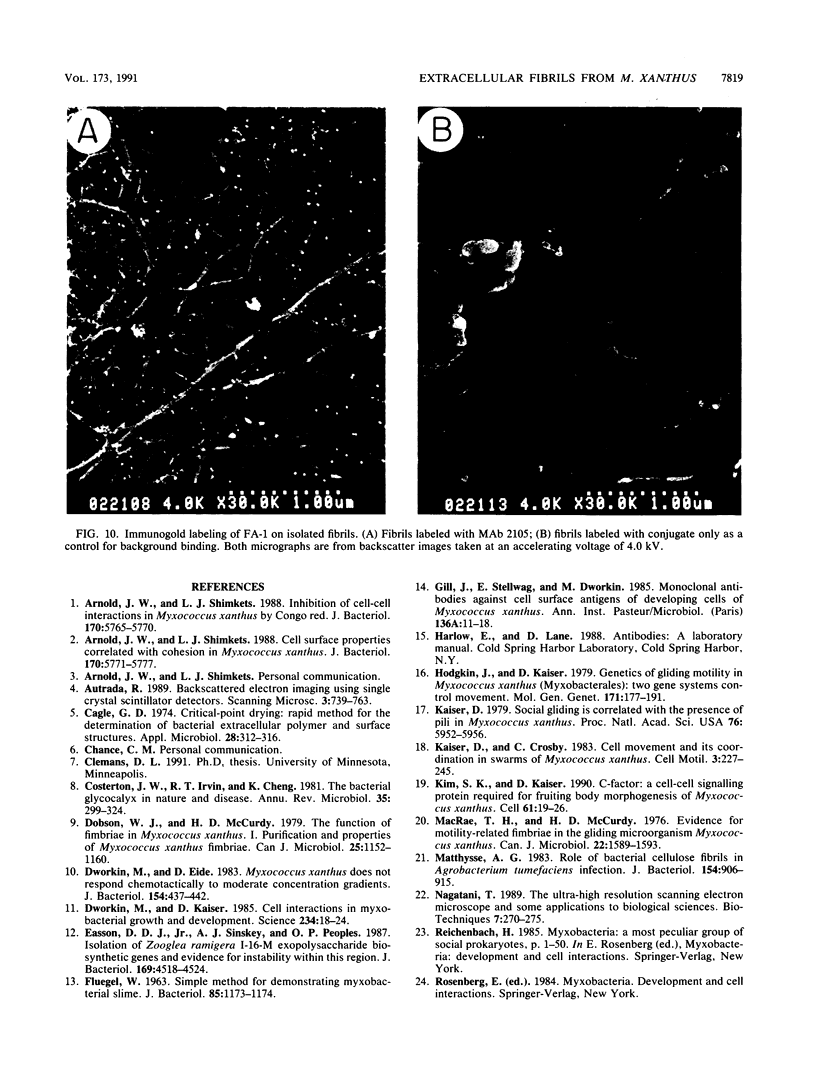
Contact-mediated cell-cell interactions play an important role in the social life-style of Myxococcus xanthus. Previous investigations have demonstrated that fimbriae (also referred to as pili) and extracellular fibrils are involved in these social interactions (L. J. Shimkets, Microbiol. Rev. 54:473-501, 1990). We have used the relatively new technique of low-voltage scanning electron microscopy (an ultra-high-resolution scanning technique that allows for the nanometer resolution of biological materials) to observe the topological details of cell-cell interactions in M. xanthus. Our observations indicated that the fibrils (which measure approximately 30 nm in diameter) are produced most extensively by cells that are in close contact with each other and are aberrantly produced by the cohesion-deficient dsp mutants. Immunogold analysis identified an antigen which is located exclusively on the extracellular fibrils. Western blots (immunoblots) of this antigen (designated FA-1 for fibrillar antigen 1) indicated that it is composed of several immunoreactive bands (molecular size range, 90 to 14 kDa), all of which are sensitive to protease digestion. A technique for fibril isolation was developed by using FA-1 as a fibril-specific marker. Low-voltage scanning electron microscope observations of swarming cells demonstrated that the expression of fibrils is differentially regulated between adventurous (individual) and socially (group) motile cells. The differential expression of fibrils suggests the existence of a mechanism for the regulation of fibril biosynthesis that functions within the overall system governing social interactions in M. xanthus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold J. W., Shimkets L. J. Cell surface properties correlated with cohesion in Myxococcus xanthus. J Bacteriol. 1988 Dec;170(12):5771–5777. doi: 10.1128/jb.170.12.5771-5777.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J. W., Shimkets L. J. Inhibition of cell-cell interactions in Myxococcus xanthus by congo red. J Bacteriol. 1988 Dec;170(12):5765–5770. doi: 10.1128/jb.170.12.5765-5770.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle G. D. Critical-point drying: rapid method for the determination of bacterial extracellular polymer and surface structures. Appl Microbiol. 1974 Aug;28(2):312–316. doi: 10.1128/am.28.2.312-316.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Dobson W. J., McCurdy H. D. The function of fimbriae in Myxococcus xanthus. I. Purification and properties of M. xanthus fimbriae. Can J Microbiol. 1979 Oct;25(10):1152–1160. doi: 10.1139/m79-179. [DOI] [PubMed] [Google Scholar]
- Dworkin M., Eide D. Myxococcus xanthus does not respond chemotactically to moderate concentration gradients. J Bacteriol. 1983 Apr;154(1):437–442. doi: 10.1128/jb.154.1.437-442.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin M., Kaiser D. Cell interactions in myxobacterial growth and development. Science. 1985 Oct 4;230(4721):18–24. doi: 10.1126/science.3929384. [DOI] [PubMed] [Google Scholar]
- Easson D. D., Jr, Sinskey A. J., Peoples O. P. Isolation of Zoogloea ramigera I-16-M exopolysaccharide biosynthetic genes and evidence for instability within this region. J Bacteriol. 1987 Oct;169(10):4518–4524. doi: 10.1128/jb.169.10.4518-4524.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLUEGEL W. SIMPLE METHOD FOR DEMONSTRATING MYXOBACTERIAL SLIME. J Bacteriol. 1963 May;85:1173–1174. doi: 10.1128/jb.85.5.1173-1174.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J., Stellwag E., Dworkin M. Monoclonal antibodies against cell-surface antigens of developing cells of Myxococcus xanthus. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):11–18. doi: 10.1016/s0769-2609(85)80015-6. [DOI] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K., Kaiser D. C-factor: a cell-cell signaling protein required for fruiting body morphogenesis of M. xanthus. Cell. 1990 Apr 6;61(1):19–26. doi: 10.1016/0092-8674(90)90211-v. [DOI] [PubMed] [Google Scholar]
- MacRae T. H., McCurdy D. Evidence for motility-related fimbriae in the gliding microorganism Myxococcus xanthus. Can J Microbiol. 1976 Oct;22(10):1589–1593. doi: 10.1139/m76-234. [DOI] [PubMed] [Google Scholar]
- Matthysse A. G. Role of bacterial cellulose fibrils in Agrobacterium tumefaciens infection. J Bacteriol. 1983 May;154(2):906–915. doi: 10.1128/jb.154.2.906-915.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani T. The ultra-high resolution scanning electron microscope and some applications to biological studies. Biotechniques. 1989 Mar;7(3):270–275. [PubMed] [Google Scholar]
- Rosenberg E., Keller K. H., Dworkin M. Cell density-dependent growth of Myxococcus xanthus on casein. J Bacteriol. 1977 Feb;129(2):770–777. doi: 10.1128/jb.129.2.770-777.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus. J Bacteriol. 1986 Jun;166(3):837–841. doi: 10.1128/jb.166.3.837-841.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J., Kaiser D. Induction of coordinated movement of Myxococcus xanthus cells. J Bacteriol. 1982 Oct;152(1):451–461. doi: 10.1128/jb.152.1.451-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Social and developmental biology of the myxobacteria. Microbiol Rev. 1990 Dec;54(4):473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit G., Kijne J. W., Lugtenberg B. J. Involvement of both cellulose fibrils and a Ca2+-dependent adhesin in the attachment of Rhizobium leguminosarum to pea root hair tips. J Bacteriol. 1987 Sep;169(9):4294–4301. doi: 10.1128/jb.169.9.4294-4301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W., Thomson S. Comparison of polysaccharides produced by Myxococcus strains. J Gen Microbiol. 1975 Jul;89(1):124–132. doi: 10.1099/00221287-89-1-124. [DOI] [PubMed] [Google Scholar]