Abstract
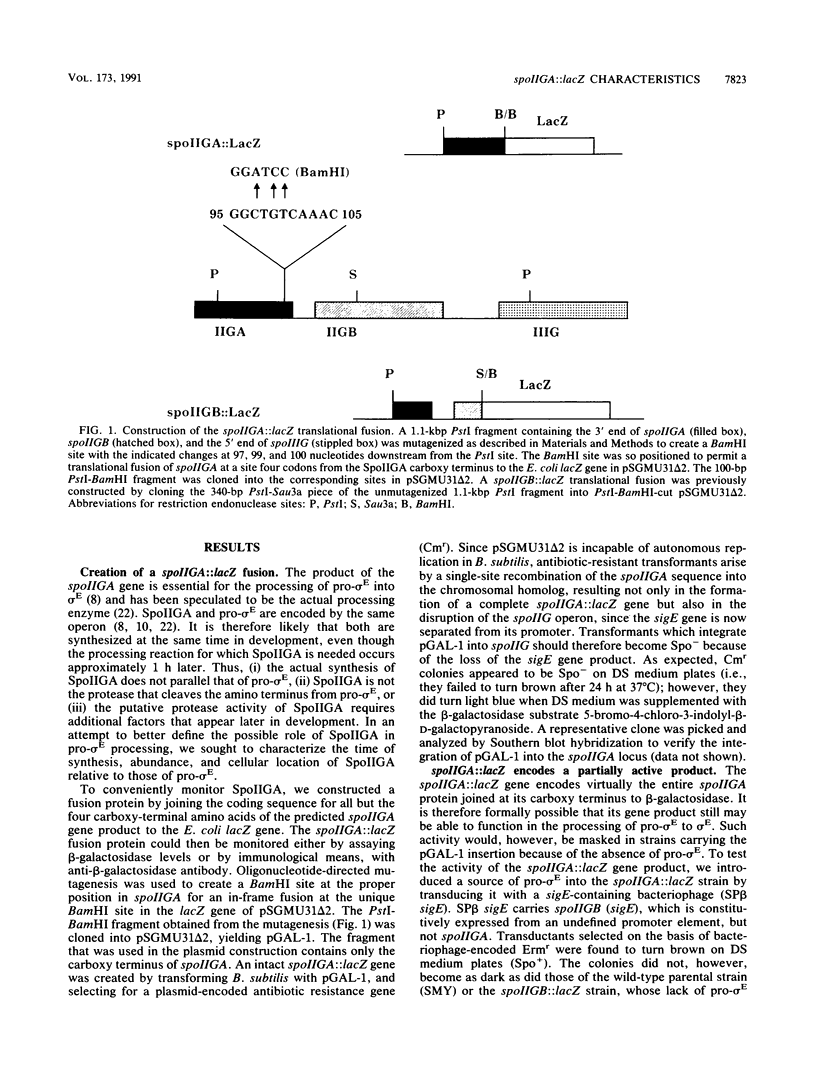
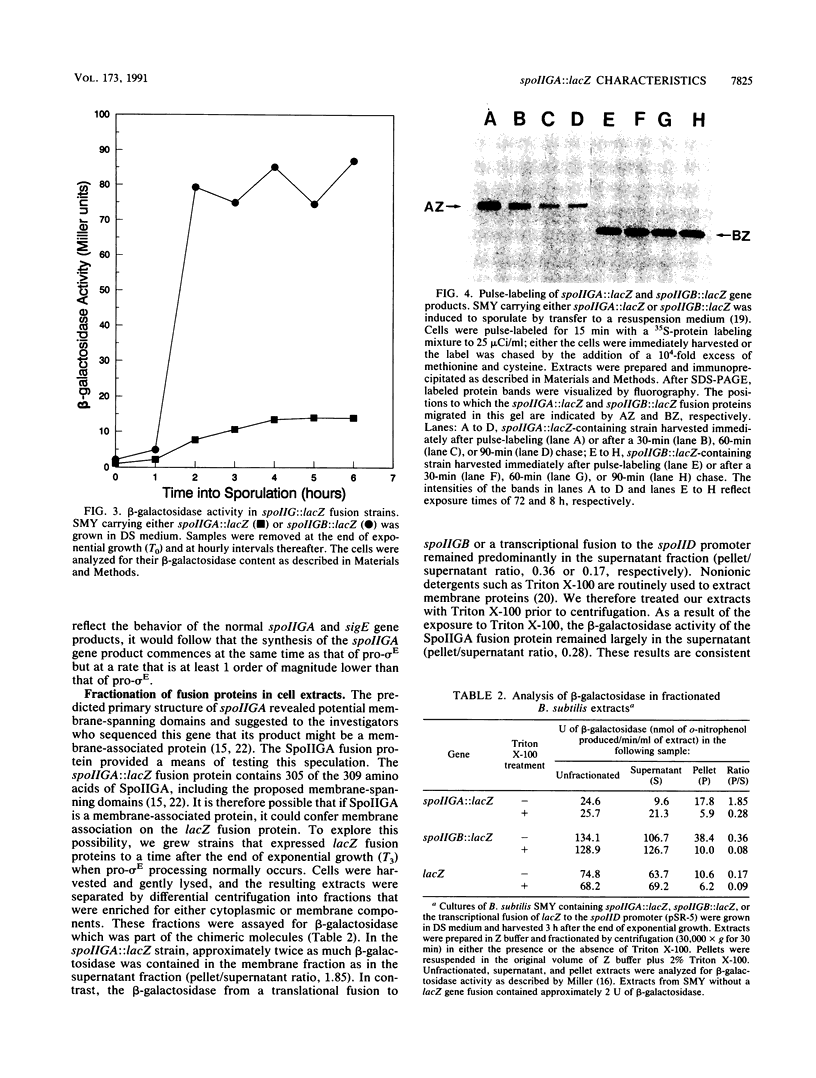
sigma E, a major sporulation-specific sigma factor of Bacillus subtilis, is derived from an inactive precursor protein (pro-sigma E). The formation of sigma E from pro-sigma E requires the products of several stage II genes, including spoIIGA, a gene that is cotranscribed with the pro-sigma E coding region (spoIIGB, or sigE). SpoIIGA has been hypothesized to be both a membrane-bound protein and the protease which converts pro-sigma E into sigma E. to learn more of its properties, we joined the Escherichia coli lacZ gene to the 3' end of spoIIGA as a translational fusion, creating a gene whose product was found to contain both beta-galactosidase and SpoIIGA activities. Assaying for the beta-galactosidase activity of the chimeric protein as a measure of its abundance, we determined that the spoIIGA::lacZ product accumulated to approximately 10% the level of a spoIIGB::lacZ fusion protein. Using differential centrifugation to fractionate B. subtilis extracts that contained beta-galactosidase fusion proteins, we observed that the beta-galactosidase activity of the spoIIGA::lacZ fusion protein was preferentially associated with a Triton X-100-sensitive, fast-sedimenting portion of the extract, while the beta-galactosidase activity of the spoIIGB::lacZ fusion protein remained primarily in the supernatant fraction. If the properties of the fusion proteins are assumed to be representative of those of the products of the genes to which lacZ is joined, these results support the hypothesis that SpoIIGA is a membrane-bound protein that acts catalytically in the processing of pro-sigma E into sigma E.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beall B., Lutkenhaus J. FtsZ in Bacillus subtilis is required for vegetative septation and for asymmetric septation during sporulation. Genes Dev. 1991 Mar;5(3):447–455. doi: 10.1101/gad.5.3.447. [DOI] [PubMed] [Google Scholar]
- Dawes I. W., Kay D., Mandelstam J. Sporulation in Bacillus subtilis. Establishment of a time scale for the morphological events. J Gen Microbiol. 1969 May;56(2):171–179. doi: 10.1099/00221287-56-2-171. [DOI] [PubMed] [Google Scholar]
- Haldenwang W. G., Lang N., Losick R. A sporulation-induced sigma-like regulatory protein from B. subtilis. Cell. 1981 Feb;23(2):615–624. doi: 10.1016/0092-8674(81)90157-4. [DOI] [PubMed] [Google Scholar]
- Illing N., Errington J. Genetic regulation of morphogenesis in Bacillus subtilis: roles of sigma E and sigma F in prespore engulfment. J Bacteriol. 1991 May;173(10):3159–3169. doi: 10.1128/jb.173.10.3159-3169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas R. M., Haldenwang W. G. Influence of spo mutations on sigma E synthesis in Bacillus subtilis. J Bacteriol. 1989 Sep;171(9):5226–5228. doi: 10.1128/jb.171.9.5226-5228.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas R. M., Holt S. C., Haldenwang W. G. Effects of antibiotics on synthesis and persistence of sigma E in sporulating Bacillus subtilis. J Bacteriol. 1990 Aug;172(8):4616–4623. doi: 10.1128/jb.172.8.4616-4623.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas R. M., Peters H. K., 3rd, Haldenwang W. G. Phenotypes of Bacillus subtilis mutants altered in the precursor-specific region of sigma E. J Bacteriol. 1990 Aug;172(8):4178–4186. doi: 10.1128/jb.172.8.4178-4186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas R. M., Weaver E. A., Kenney T. J., Moran C. P., Jr, Haldenwang W. G. The Bacillus subtilis spoIIG operon encodes both sigma E and a gene necessary for sigma E activation. J Bacteriol. 1988 Feb;170(2):507–511. doi: 10.1128/jb.170.2.507-511.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney T. J., Kirchman P. A., Moran C. P., Jr Gene encoding sigma E is transcribed from a sigma A-like promoter in Bacillus subtilis. J Bacteriol. 1988 Jul;170(7):3058–3064. doi: 10.1128/jb.170.7.3058-3064.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney T. J., Moran C. P., Jr Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3329–3339. doi: 10.1128/jb.169.7.3329-3339.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBell T. L., Trempy J. E., Haldenwang W. G. Sporulation-specific sigma factor sigma 29 of Bacillus subtilis is synthesized from a precursor protein, P31. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1784–1788. doi: 10.1073/pnas.84.7.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Youngman P., Piggot P. J. Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet. 1986;20:625–669. doi: 10.1146/annurev.ge.20.120186.003205. [DOI] [PubMed] [Google Scholar]
- Masuda E. S., Anaguchi H., Sato T., Takeuchi M., Kobayashi Y. Nucleotide sequence of the sporulation gene spoIIGA from Bacillus subtilis. Nucleic Acids Res. 1990 Feb 11;18(3):657–657. doi: 10.1093/nar/18.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Rong S., Rosenkrantz M. S., Sonenshein A. L. Transcriptional control of the Bacillus subtilis spoIID gene. J Bacteriol. 1986 Mar;165(3):771–779. doi: 10.1128/jb.165.3.771-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stragier P., Bonamy C., Karmazyn-Campelli C. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell. 1988 Mar 11;52(5):697–704. doi: 10.1016/0092-8674(88)90407-2. [DOI] [PubMed] [Google Scholar]
- Stragier P., Bouvier J., Bonamy C., Szulmajster J. A developmental gene product of Bacillus subtilis homologous to the sigma factor of Escherichia coli. Nature. 1984 Nov 22;312(5992):376–378. doi: 10.1038/312376a0. [DOI] [PubMed] [Google Scholar]
- Trempy J. E., Bonamy C., Szulmajster J., Haldenwang W. G. Bacillus subtilis sigma factor sigma 29 is the product of the sporulation-essential gene spoIIG. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4189–4192. doi: 10.1073/pnas.82.12.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempy J. E., Morrison-Plummer J., Haldenwang W. G. Synthesis of sigma 29, an RNA polymerase specificity determinant, is a developmentally regulated event in Bacillus subtilis. J Bacteriol. 1985 Jan;161(1):340–346. doi: 10.1128/jb.161.1.340-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff D. L., Mahoney M., Shatzman A., Rosenberg M. Mutational analysis of a regulatory region in bacteriophage lambda that has overlapping signals for the initiation of transcription and translation. Proc Natl Acad Sci U S A. 1984 Jan;81(2):555–559. doi: 10.1073/pnas.81.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]




