Abstract
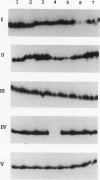
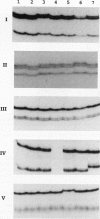
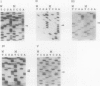
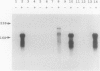
Previously, we isolated and characterized six Bacillus subtilis ada mutants that were hypersensitive to methylnitroso compounds and deficient in the adaptive response to alkylation. Cloning of the DNA complementing the defects revealed the presence of an ada operon consisting of two tandem and partially overlapping genes, adaA and adaB. The two genes encoded proteins with methylphosphotriester-DNA methyltransferase and O6-methylguanine-DNA methyltransferase activities, respectively. To locate the six mutations, the ada operon was divided into five overlapping regions of about 350 bp. The fragments of each region were amplified by polymerase chain reaction and analyzed by gel electrophoresis to detect single-strand conformation polymorphism. Nucleotide sequences of the fragments exhibiting mobility shifts were determined. Three of the mutants carried sequence alterations in the adaA gene: the adaA1 and adaA2 mutants had a one-base deletion and insertion, respectively, and the adaA5 mutant had a substitution of two consecutive bases causing changes of two amino acid residues next to the presumptive alkyl-accepting Cys-85 residue. Three mutants carried sequence alterations in the adaB gene: the adaB3 mutant contained a rearrangement, the adaB6 mutant contained a base substitution causing a change of the presumptive alkyl-accepting Cys-141 to Tyr, and the adaB4 mutant contained a base substitution changing Leu-167 to Pro. The adaB mutants produced ada transcripts upon treatment with low doses of alkylating agents, whereas the adaA mutant did not. We conclude that the AdaA protein functions as the transcriptional activator of this operon, while the AdaB protein specializes in repair of alkylated residues in DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Clarke P., Lin H. C., Wilcox G. The nucleotide sequence of the araC regulatory gene in Salmonella typhimurium LT2. Gene. 1982 May;18(2):157–163. doi: 10.1016/0378-1119(82)90113-5. [DOI] [PubMed] [Google Scholar]
- Demple B., Sedgwick B., Robins P., Totty N., Waterfield M. D., Lindahl T. Active site and complete sequence of the suicidal methyltransferase that counters alkylation mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(9):2688–2692. doi: 10.1073/pnas.82.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa H., Koike G., Sekiguchi M. Expression and cloning of complementary DNA for a human enzyme that repairs O6-methylguanine in DNA. J Mol Biol. 1990 Jun 20;213(4):739–747. doi: 10.1016/S0022-2836(05)80260-8. [DOI] [PubMed] [Google Scholar]
- Hughes S. J., Sedgwick B. The adaptive response to alkylation damage. Constitutive expression through a mutation in the coding region of the ada gene. J Biol Chem. 1989 Dec 15;264(35):21369–21375. [PubMed] [Google Scholar]
- Ikawa S., Shibata T., Matsumoto K., Iijima T., Saito H., Ando T. Chromosomal loci of genes controlling site-specific restriction endonucleases of Bacillus subtilis. Mol Gen Genet. 1981;183(1):1–6. doi: 10.1007/BF00270129. [DOI] [PubMed] [Google Scholar]
- Inouye S., Nakazawa A., Nakazawa T. Nucleotide sequence of the regulatory gene xylS on the Pseudomonas putida TOL plasmid and identification of the protein product. Gene. 1986;44(2-3):235–242. doi: 10.1016/0378-1119(86)90187-3. [DOI] [PubMed] [Google Scholar]
- Jeggo P. Isolation and characterization of Escherichia coli K-12 mutants unable to induce the adaptive response to simple alkylating agents. J Bacteriol. 1979 Sep;139(3):783–791. doi: 10.1128/jb.139.3.783-791.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama K. I., Nakabeppu Y., Sekiguchi M. Cloning and expression of the Bacillus subtilis methyltransferase gene in Escherichia coli ada- cells. Mutat Res. 1989 Sep;218(2):153–163. doi: 10.1016/0921-8777(89)90022-0. [DOI] [PubMed] [Google Scholar]
- Kondo H., Nakabeppu Y., Kataoka H., Kuhara S., Kawabata S., Sekiguchi M. Structure and expression of the alkB gene of Escherichia coli related to the repair of alkylated DNA. J Biol Chem. 1986 Nov 25;261(33):15772–15777. [PubMed] [Google Scholar]
- Lemotte P. K., Walker G. C. Induction and autoregulation of ada, a positively acting element regulating the response of Escherichia coli K-12 to methylating agents. J Bacteriol. 1985 Mar;161(3):888–895. doi: 10.1128/jb.161.3.888-895.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Sedgwick B., Sekiguchi M., Nakabeppu Y. Regulation and expression of the adaptive response to alkylating agents. Annu Rev Biochem. 1988;57:133–157. doi: 10.1146/annurev.bi.57.070188.001025. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Cooper D. P., Brennand J. Cloning of the E. coli O6-methylguanine and methylphosphotriester methyltransferase gene using a functional DNA repair assay. Nucleic Acids Res. 1985 Mar 25;13(6):1939–1952. doi: 10.1093/nar/13.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy T. V., Karran P., Lindahl T. Inducible repair of O-alkylated DNA pyrimidines in Escherichia coli. EMBO J. 1984 Mar;3(3):545–550. doi: 10.1002/j.1460-2075.1984.tb01844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyada C. G., Horwitz A. H., Cass L. G., Timko J., Wilcox G. DNA sequence of the araC regulatory gene from Escherichia coli B/r. Nucleic Acids Res. 1980 Nov 25;8(22):5267–5274. doi: 10.1093/nar/8.22.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohoshi F., Hayashi K., Munakata N. Bacillus subtilis ada operon encodes two DNA alkyltransferases. Nucleic Acids Res. 1990 Sep 25;18(18):5473–5480. doi: 10.1093/nar/18.18.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohoshi F., Hayashi K., Munakata N. Bacillus subtilis gene coding for constitutive O6-methylguanine-DNA alkyltransferase. Nucleic Acids Res. 1989 Aug 25;17(16):6531–6543. doi: 10.1093/nar/17.16.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohoshi F., Munakata N. Bacillus subtilis mutants deficient in the adaptive response to simple alkylating agents. J Bacteriol. 1985 Mar;161(3):825–830. doi: 10.1128/jb.161.3.825-830.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohoshi F., Munakata N. Multiple species of Bacillus subtilis DNA alkyltransferase involved in the adaptive response to simple alkylating agents. J Bacteriol. 1987 Feb;169(2):587–592. doi: 10.1128/jb.169.2.587-592.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohoshi F., Munakata N. Two classes of Bacillus subtilis mutants deficient in the adaptive response to simple alkylating agents. Mol Gen Genet. 1986 Feb;202(2):200–206. doi: 10.1007/BF00331637. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Kondo H., Kawabata S., Iwanaga S., Sekiguchi M. Purification and structure of the intact Ada regulatory protein of Escherichia coli K12, O6-methylguanine-DNA methyltransferase. J Biol Chem. 1985 Jun 25;260(12):7281–7288. [PubMed] [Google Scholar]
- Nakabeppu Y., Sekiguchi M. Regulatory mechanisms for induction of synthesis of repair enzymes in response to alkylating agents: ada protein acts as a transcriptional regulator. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6297–6301. doi: 10.1073/pnas.83.17.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- Potter P. M., Wilkinson M. C., Fitton J., Carr F. J., Brennand J., Cooper D. P., Margison G. P. Characterisation and nucleotide sequence of ogt, the O6-alkylguanine-DNA-alkyltransferase gene of E. coli. Nucleic Acids Res. 1987 Nov 25;15(22):9177–9193. doi: 10.1093/nar/15.22.9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Sedgwick B. Molecular cloning of a gene which regulates the adaptive response to alkylating agents in Escherichia coli. Mol Gen Genet. 1983;191(3):466–472. doi: 10.1007/BF00425764. [DOI] [PubMed] [Google Scholar]
- Sedgwick B., Robins P. Isolation of mutants of Escherichia coli with increased resistance to alkylating agents: mutants deficient in thiols and mutants constitutive for the adaptive response. Mol Gen Genet. 1980;180(1):85–90. doi: 10.1007/BF00267355. [DOI] [PubMed] [Google Scholar]
- Sedgwick B., Robins P., Totty N., Lindahl T. Functional domains and methyl acceptor sites of the Escherichia coli ada protein. J Biol Chem. 1988 Mar 25;263(9):4430–4433. [PubMed] [Google Scholar]
- Suzuki Y., Orita M., Shiraishi M., Hayashi K., Sekiya T. Detection of ras gene mutations in human lung cancers by single-strand conformation polymorphism analysis of polymerase chain reaction products. Oncogene. 1990 Jul;5(7):1037–1043. [PubMed] [Google Scholar]
- Suzuki Y., Sekiya T., Hayashi K. Allele-specific polymerase chain reaction: a method for amplification and sequence determination of a single component among a mixture of sequence variants. Anal Biochem. 1991 Jan;192(1):82–84. doi: 10.1016/0003-2697(91)90188-y. [DOI] [PubMed] [Google Scholar]
- Tano K., Shiota S., Collier J., Foote R. S., Mitra S. Isolation and structural characterization of a cDNA clone encoding the human DNA repair protein for O6-alkylguanine. Proc Natl Acad Sci U S A. 1990 Jan;87(2):686–690. doi: 10.1073/pnas.87.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo I., Sedgwick B., Kilpatrick M. W., McCarthy T. V., Lindahl T. The intracellular signal for induction of resistance to alkylating agents in E. coli. Cell. 1986 Apr 25;45(2):315–324. doi: 10.1016/0092-8674(86)90396-x. [DOI] [PubMed] [Google Scholar]
- Triglia T., Peterson M. G., Kemp D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988 Aug 25;16(16):8186–8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]